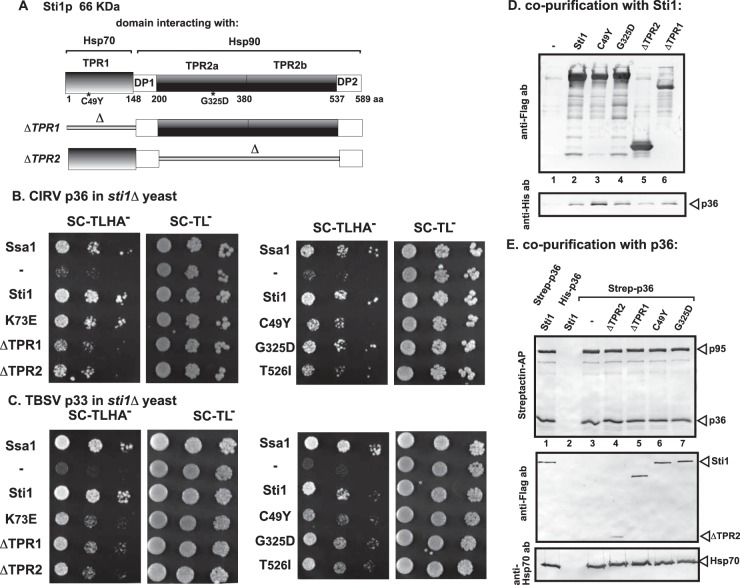
FIG 3.
Interaction between Sti1p and CIRV p36 replication protein in yeast and in vitro. (A) Domain structure of the yeast Sti1p. Tetratricopeptide repeat 1 (TPR1) sequence interacts with Hsp70, while dipeptide repeat of aspartic acid and proline 1 (DP1) might stabilize the bound client protein. TPR2A and TPR2B bind to Hsp90 and together inhibit the ATPase activity of Hsp90. TPR2B also binds to Hsp70, but only in concert with Hsp90 binding to TPR2A. The debilitating mutations are marked with an asterisk, and deletion constructs are shown schematically at the bottom of the panel. (B) A split-ubiquitin MYTH assay was used to test intracellular interaction between CIRV p36 and the wt or mutated yeast Sti1p. The bait p36 was coexpressed with the prey Sti1p protein in sti1Δ yeast. The SSA1 gene (Hsp70 chaperone) and the empty prey vector (NubG) were used as positive and negative controls, respectively. (C) Same split-ubiquitin MYTH assay as that shown in panel B, except that TBSV p33 was used as a bait protein. (D) Copurification of CIRV p36 replication protein with the yeast Sti1p from yeast cells. The membrane fraction of yeast coexpressing the wt or mutated FLAG-Sti1p and His6-p36 was solubilized, and the Sti1p variants were purified using a FLAG column. The eluted proteins were tested using Western blotting with anti-FLAG antibody (top image) and anti-His6 antibody (bottom image). (E) Reciprocal copurification of the yeast Sti1p with CIRV p36 and p95 replication proteins from yeast cells. Details are as described for panel D, except yeast coexpressed the Twin-Strep-tagged CIRV p36 and p95 and Flag-Sti1p. The purification was based on Strep-Tactin columns. The eluted proteins were tested using Western blotting with anti-Strep-Tactin-AP conjugate (top image), anti-Flag antibody (middle image), and anti-Hsp70 antibody (bottom image). Note that the coopted Hsp70 is a permanent member of the tombusvirus replicase complex. Each experiment was repeated three times.