FIG 5.
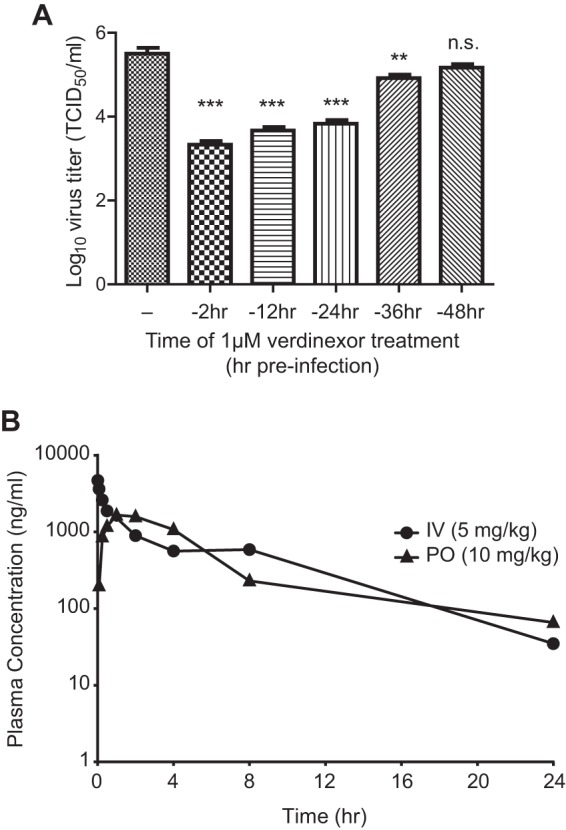
Verdinexor is a slowly reversible inhibitor of XPO1-mediated influenza virus replication in vitro and is bioavailable after oral administration in vivo. (A) A549 cells were mock treated (−) or treated with 1 μM verdinexor for 2 h. Following treatment, the drug-containing medium was removed and replaced with normal growth medium. Cells were incubated for different lengths of time posttreatment, prior to infection with influenza virus A/WSN/33 at an MOI of 0.01. Culture supernatants were collected at 48 hpi for virus titer determination. Data are expressed as the mean ± SE (n = 3). n.s., not significant; **, P < 0.01; ***, P < 0.001 (relative to mock-treated infected cells). (B) For a PK study of p.o. or i.v. administration of verdinexor in mice, verdinexor was given orally by gavage at 10 mg/kg (30 mg/m2) or i.v. at 5 mg/kg (15 mg/m2). Plasma verdinexor concentrations were determined with a validated ultrahigh-performance liquid chromatography–mass spectrometry method, and the mean concentration at each time point postadministration was plotted (n = 3).
