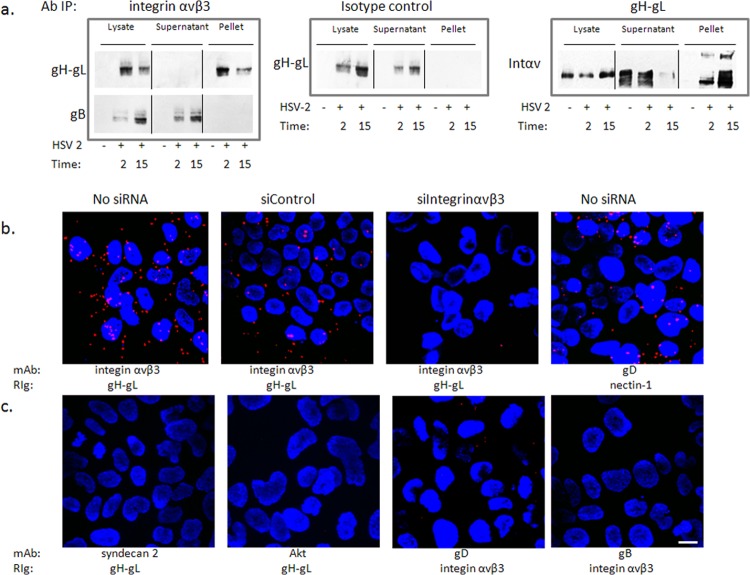
FIG 4.
Integrin αvβ3 interacts with glycoprotein H. (a) CaSki cells were synchronously infected with purified HSV-2 (5 PFU/cell), and cell lysates were harvested 2 and 15 min post-temperature shift and incubated with monoclonal mouse anti-integrin αvβ3 (left panels) or an isotype control MAb (middle panel). Immune complexes were precipitated, and equivalent volumes of the whole-cell lysate (starting material), supernatant, and pellet were subjected to Western blotting with rabbit anti-gH-gL (upper left) or goat anti-gB (lower left) antibodies. In reciprocal experiments, lysates were precipitated with monoclonal antibodies to gH-gL and analyzed by Western blotting with rabbit polyclonal antibodies to integrin αv (right panel). Controls included uninfected cell lysates; blots shown are representative of 5 independent experiments. (b) CaSki cells were synchronously infected with purified HSV-2(G) (5 PFU/cell) (no siRNA) 72 h after being transfected with the indicated siRNA. The cells were subsequently fixed and probed with monoclonal mouse antibodies (MAb) to integrin αvβ3 or gD and rabbit sera (RIg) to gH-gL or nectin-1 and assessed in a proximity ligation assay. (c) Additional proximity ligation studies were conducted with nontransfected CaSki cells that were synchronously infected with HSV-2(G) as in panel b and fixed and probed with the indicated antibodies. Proximity ligation results are representative of 2 independent experiments. Bar = 10 μM.