FIG 1.
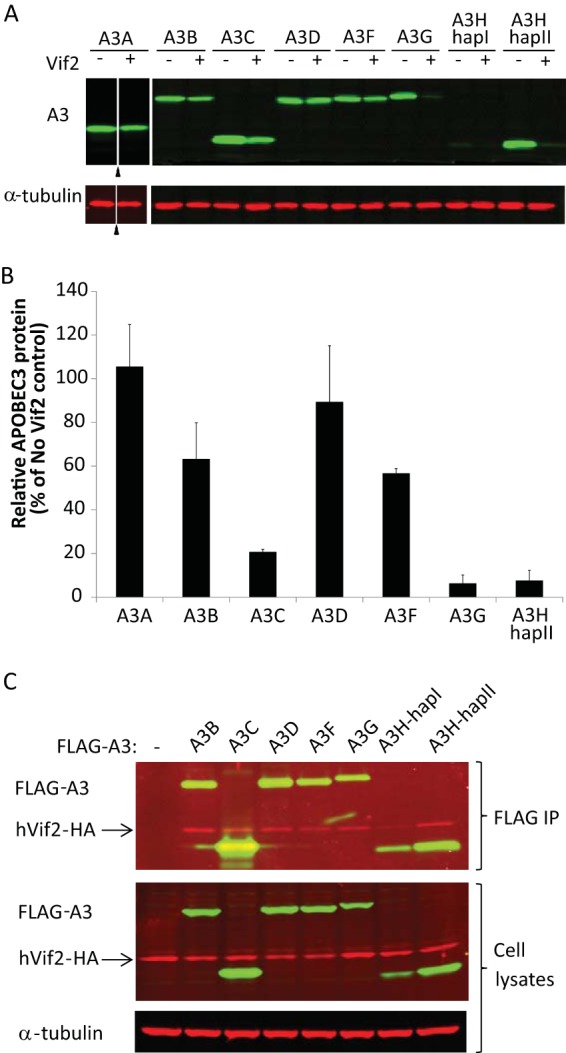
Interactions between human APOBEC3 proteins and HIV-2 Vif. HEK293T cells that were cotransfected with A3 expression plasmids and either empty vector or Vif2 expression plasmids were analyzed. (A) Sensitivity of human A3 proteins to Vif2. Cell lysates were analyzed by quantitative Western blotting using an anti-myc antibody to detect myc-A3A, an anti-FLAG antibody to detect FLAG-A3B, -A3C, -A3D, -A3F, -A3G, -A3H-hapI, and -A3H-hapII, or an anti-α-tubulin antibody to detect α-tubulin. Triangles, lanes derived from the same blot with extraneous lanes were removed; lanes +, with Vif2; lanes −, without Vif2. (B) The A3 band intensities were normalized by the α-tubulin intensities, and the relative A3 protein levels (FLAG or myc) in the presence of Vif2 were calculated as a percentage of the amount of A3 protein in the absence of Vif2 (set equal to 100% for each A3 protein). A3H-hapI was not quantitated due to the low signal intensity in the absence of Vif2. The average of two independent experiments is shown; error bars indicate standard deviations. (C) Co-IP assays to determine binding of Vif2 to A3 proteins. HEK293T cells were cotransfected with FLAG-A3 expression plasmids and hVif2-HA expression plasmids. Cell lysates and immunoprecipitated proteins were analyzed by Western blotting using anti-FLAG and anti-HA antibodies to detect FLAG-A3 and hVif2-HA proteins, respectively. α-Tubulin was also detected in the cell lysates as a loading control.
