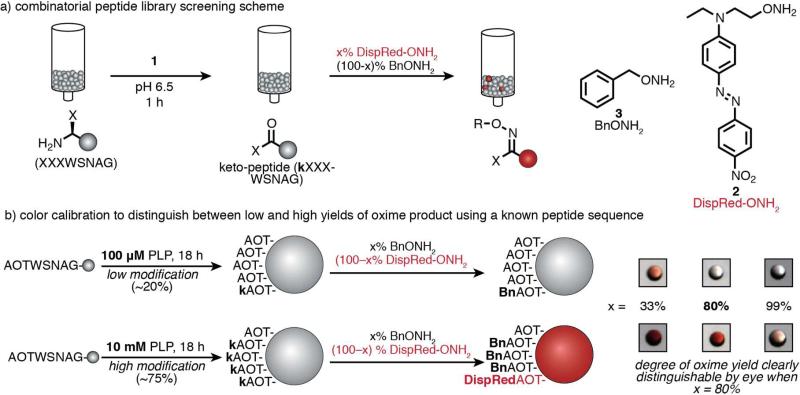
Figure 2.
(a) A one-bead-one-sequence combinatorial peptide library in which the three N-terminal residues were varied was used to screen potential transamination reagents (1) against all possible N-terminal sequences simultaneously. After transamination, the keto-group-containing peptides were identified through the covalent attachment of a visible dye (DispRed-ONH2, 2) through oxime formation. (b) To adjust the sensitivity of the colorimetric detection method, a colorless alkoxyamine (BnONH2, 3) was combined with DispRed-ONH2 in varying ratios. A peptide sequence (AOT-terminal, with similar reactivity to AKT-termini) and a transamination reagent with known reactivity (PLP, 1a) were used to generate beads with high (75%) and low (20%) levels of modification. The greatest visual distinction between beads bearing high and low modification levels was observed when using 80:20 ratio of 3 to 2.