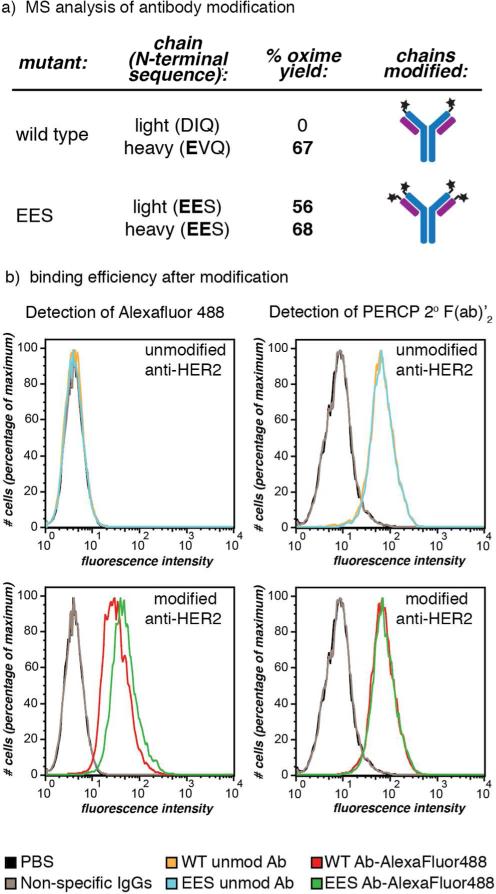
Figure 5.
IgG modification using RS-mediated transamination. (a) Wild-type anti-HER2 human IgG1 antibodies, as well as mutants with EES N-terminal sequences, were treated with RS, followed by oxime formation with BnONH2. The heavy and light chains were separated for mass spectrometry quantification of the benzyl oxime product. The wild-type antibody showed modification of the heavy chain and no modification of the light chain, while the mutant with EES on both sets of termini exhibited modification of both chains. (b) The antigen binding ability of wild-type (WT) and EES-terminal anti-HER2 antibodies post-modification (transaminated by RS, followed by oxime formation with AlexaFluor488-ONH2) was confirmed using flow cytometry. MCF7 clone 18 cells (a HER2 positive cell line) were subjected to modified (bottom histograms) and unmodified (top) wild-type and EES-terminal antibodies. By direct detection of the AlexaFluor488, the binding of modifed antibodies was seen (bottom left), while the unmodified showed no fluorescent shift (top left). In order to compare the binding of the modified antibodies to unmodified, the unmodified species were detected using a secondary detection method: anti-human IgG PerCP-conjugated 2° F(ab’)2 (right histograms). Both wild-type and EES-terminal antibodies modified with the fluorophore retained similar binding affinity and specificity to the unmodified ones. Non-specific human IgG1 and PBS were used as negative control agents. Jurkat cells (a HER2 negative cell line) did not bind to either unmodified or modified anti-HER2 antibodies (Figure S15).