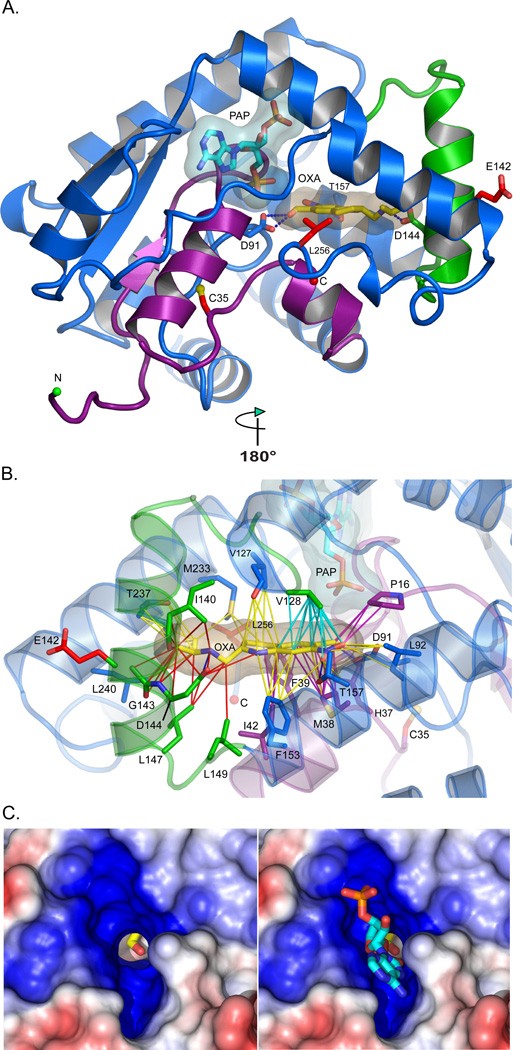
Fig. 3. Structure of Smp_089320 sulfotransferase.
(A) Smp_089320 sulfotransferase structure refined at 1.75 Å resolution. The depleted co-factor PAP and the pro-drug OXA are cyan and yellow sticks with semi-transparent envelopes as van der Waals surfaces. Residues 1–45 (purple) are major structural determinants of PAP and OXA binding. C35, E142, and L256 mutation sites are red sticks. D91, D144, and T157 (labeled) form hydrogen bonds (blue dashes) with OXA. (B) Contacts likely disrupted by the C35R and E142Δ mutations are purple and red lines, respectively. Cyan contacts could be compromised (Table S5). (C) Positive (blue) and negative (red) electrostatic potentials contoured at +/− 5kT around the PAP binding site. The OXA hydroxyl group to be sulfonated (Fig. S1) is visible in the PAPS 5′-phosphosulfate access shaft without (left) and with (right) PAP. The shaft permits the PAPS 5'-phosphosulfate to project into the cavity where the OXA hydroxyl group resides (Fig. S1).