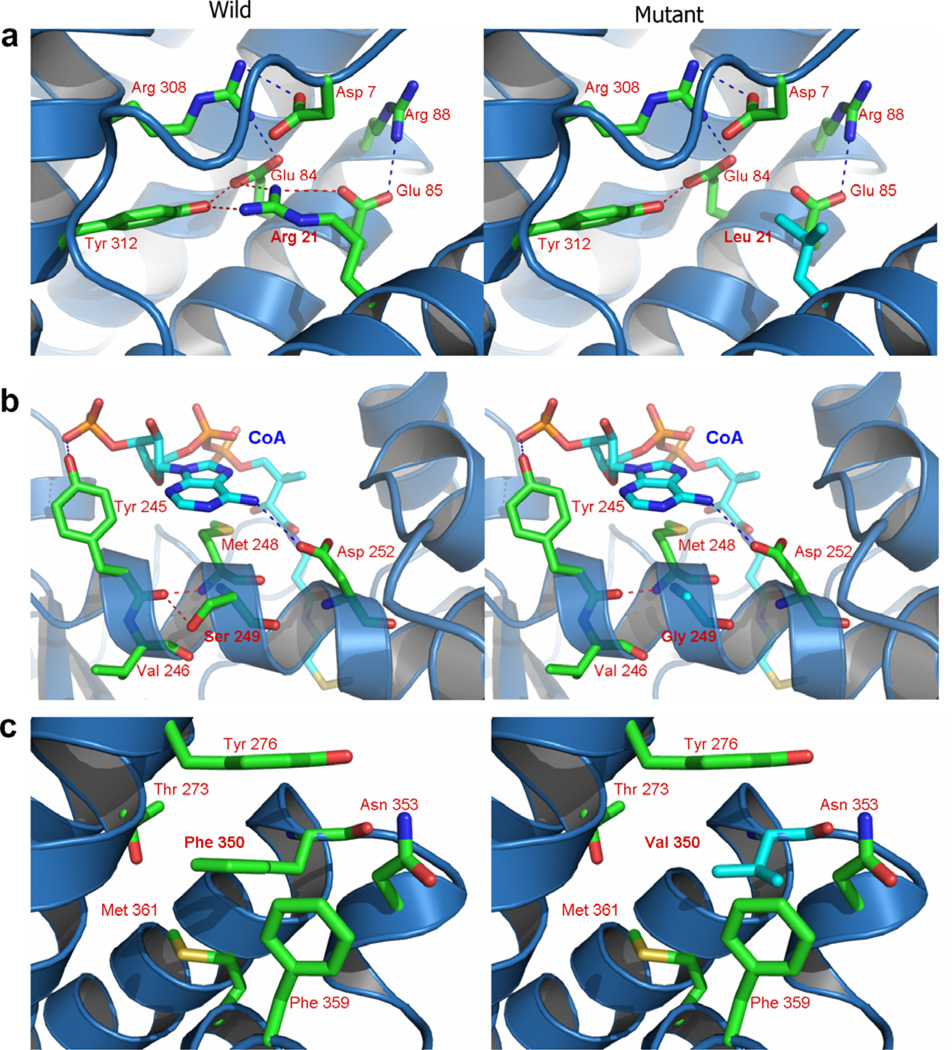
Fig. 4.
Predicted structural changes in human IVD induced by patient mutations. (a) Changing of Arg21 to leucine is predicted to induce a conformation change in α-helix A, weakening the salt bridging between Arg21 and disrupting the hydrogen bond between Arg21 and Tyr312. (b) The Ser249Gly, is predicted to eliminate a hydrogen bond normally present between Ser249 with Val246 increasing flexibility of the α-helix associated with the IVD catalytic site. (c) In the Phe350Val, hydrophobic interactions among Tyr276, Met361 and Phe359 are predicted to be weakened and π–π interaction between Phe350 and Tyr276 decreased.