Figure 1. Electron microscopy reveals aggresome-like α globin inclusions in β-thalassemia.
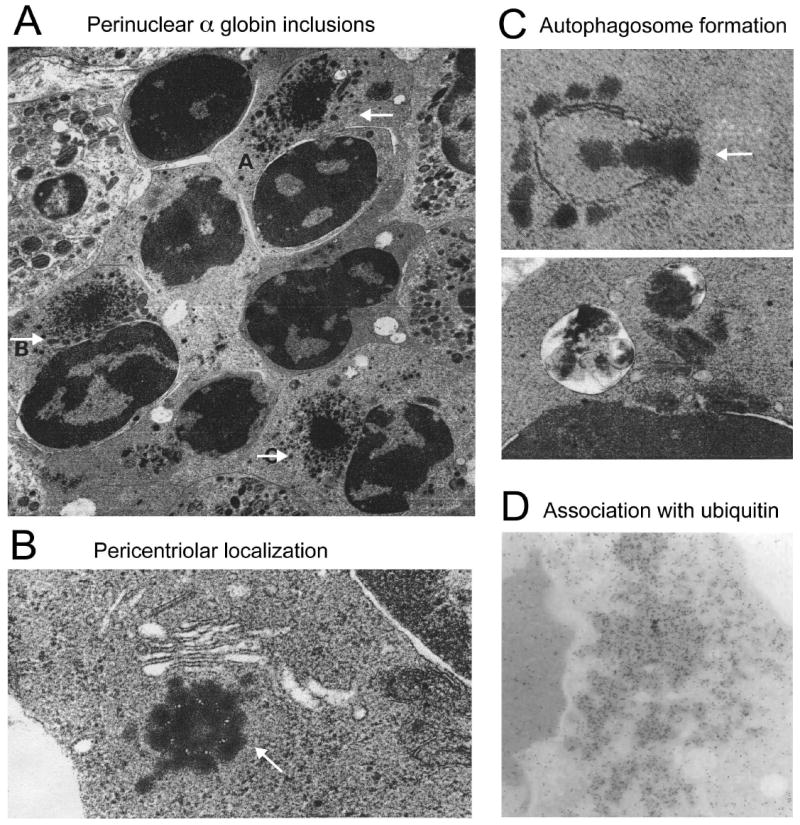
(A) Several late polychromatic erythroblasts, labeled A, B, and C, with intracytoplasmic perinuclear α-chain precipitates (adapted from Wickramasinghe SN, Bush V. Observations on the ultrastructure of erythropoietic cells and reticulum cells in the bone marrow of patients with homozygous beta-thalassaemia. Br J Haematol 1975;30(4):395-399, with permission); (B) precipitation of α-chains around the centriole of an erythroblast. The arrow indicates microtubule triplets in centriole cross-section (adapted from Wickramasinghe SN, Hughes M. Precipitation of alpha-chains on the centrioles of erythroblasts in beta-thalassaemia. Br J Haematol 1982;52(4):681-682, with permission); (C) Top: Autophagosome membrane formation partially enclosing precipitated α-chains; Bottom: Two autophagic vacuoles containing electrodense material, probably precipitated α chains (adapted from Wickramasinghe SN, Hughes M. Ultrastructural studies of erythropoiesis in beta-thalassaemia trait. Br J Haematol 1980;46(3):401-407, with permission); (D) Immunogold staining for ubiquitin within α globin precipitates of a β-thalassemic erythroblast (adapted from Wickramasinghe SN, Lee MJ. Evidence that the ubiquitin proteolytic pathway is involved in the degradation of precipitated globin chains in thalassaemia. Br J Haematol 1998;101(2):245-250, with permission).
