Table 6.
Transformation of Trichloroacetamide 14 to α-Urea Glycosides with Amino Acid and Carbohydrate Amine Nucleophiles.[a]

| |||
|---|---|---|---|
| Entry | Amines | Ureas | Yield (%)[b] |
| 1 |
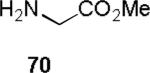
|

|
85 |
| 2 |

|

|
80 |
| 3 |

|

|
90 |
| 4 |

|

|
66 |
| 5 |

|

|
81 |
All urea-forming reactions were performed with 2-3 equiv. amine and 3-5 equiv. Cs2CO3 at 25 °C in DMF (0.2 M).
Isolated Yield.
