Figure 5.
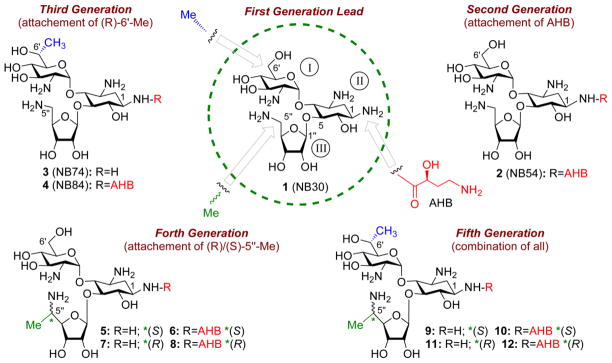
Chronological development of lead compounds on the pseudo-trisaccharide (compound 1) scaffold by systematic optimization of structure-activity-toxicity relationship as follow: first-generation lead (compound 1, also called NB30) 68 was developed by attaching 5-amino ribose at C5 position of the paromamine (rings I and II of paromomycin); second-generation compound 2 (also called NB54), 69 by further attaching (S)-4-amino-2-hydroxybutanoyl (AHB) group at N-1 position of the compound 1; third-generation compounds 3 and 4 (also named NB74 and NB84, respectively), 70 by installing (R)-6'-Me on 1 and 2, respectively; fourth-generation compounds 5–8, 66 by attaching (S)/(R)-5''-Me on either 1 or 2; fifth-generation compounds 9–12, 59 by installing (S)/(R)-5''-Me on either 3 or 4. The identity of each pharmacophore and its attachment site are highlighted: AHB, red; (R)-6'-Me, blue; (S)/(R)-5''-Me, green.
