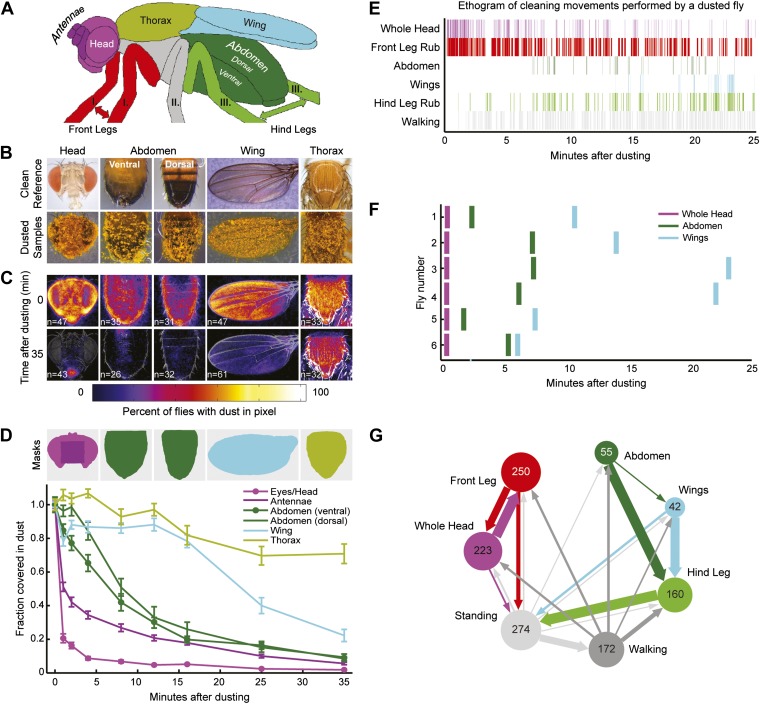
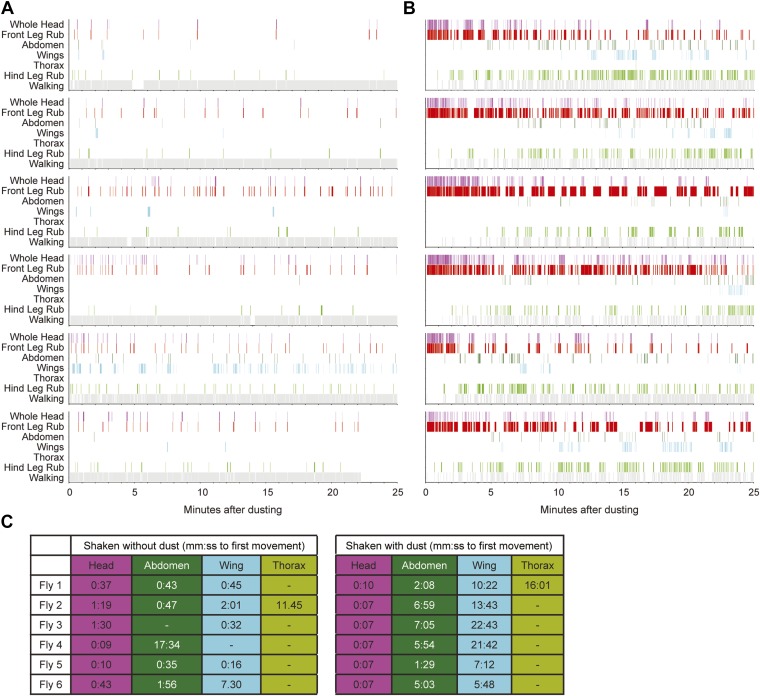
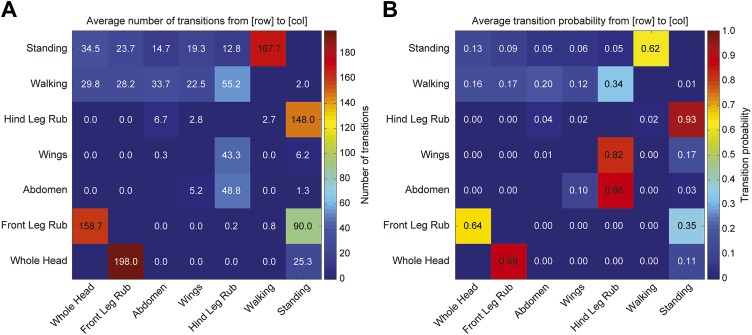
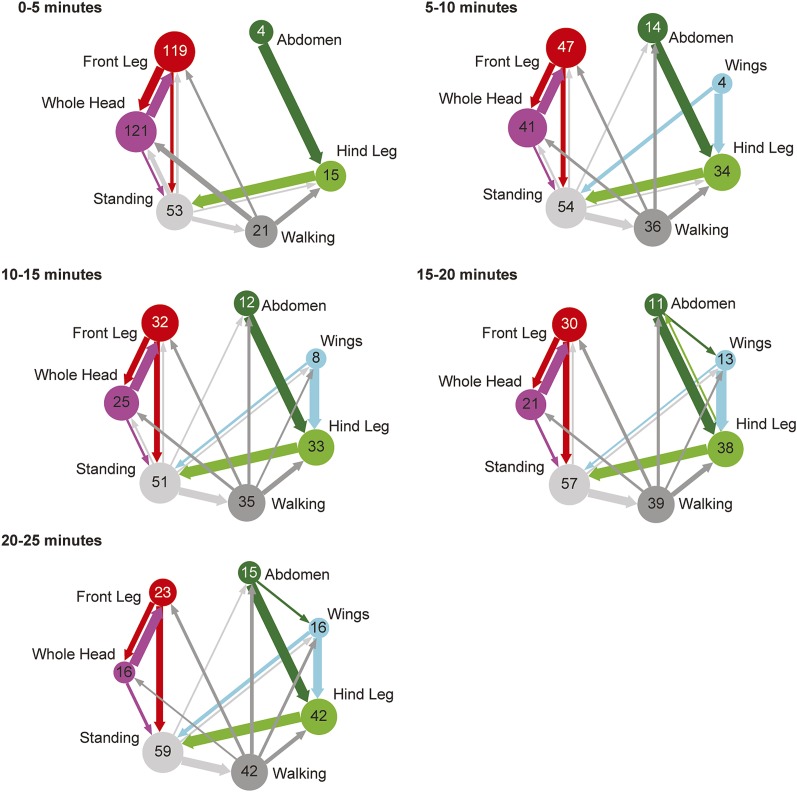
Figure 1. Wild-type flies clean different areas of the body sequentially.
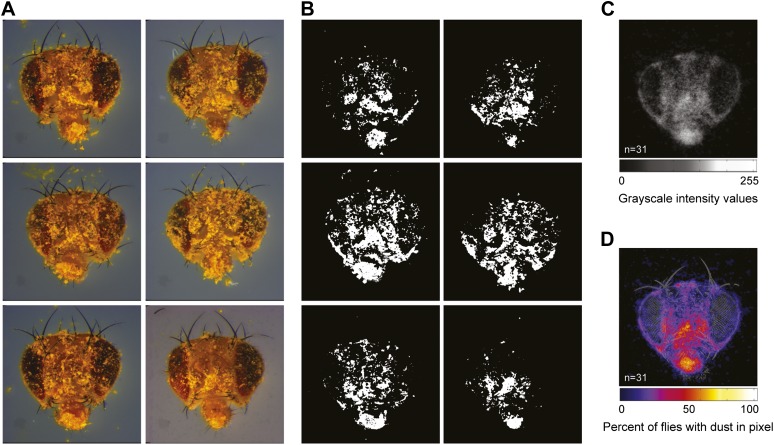
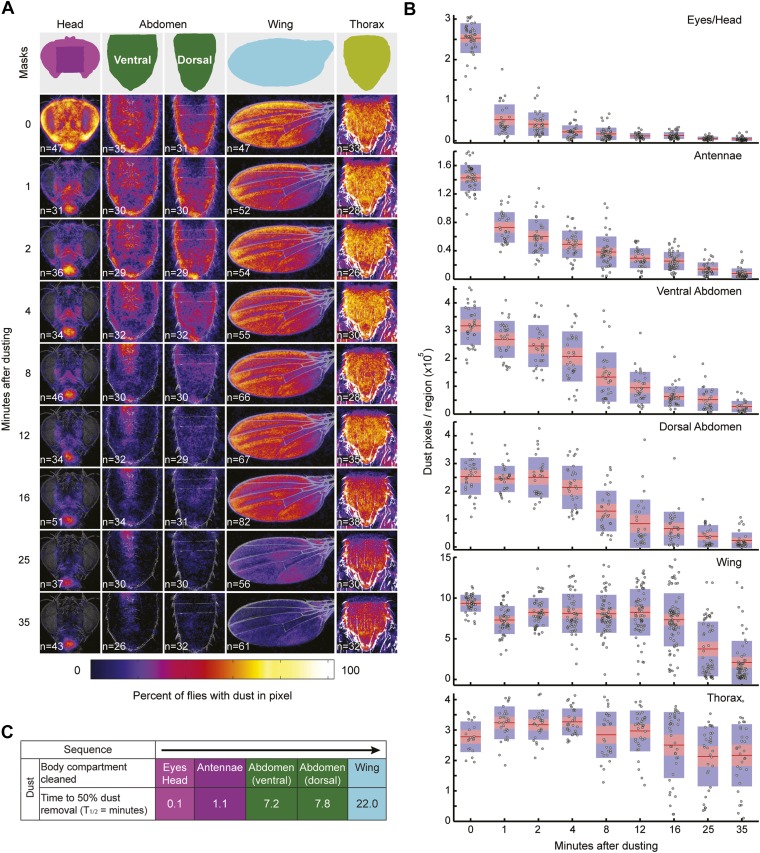
(A) Diagram of body parts cleaned by front leg (red hues) or hind leg (green hues) movements. (B–D) Dust distribution measurements of the bodies of flies that were coated in yellow dust and allowed to groom. (B) Body parts were imaged (dusted samples) and aligned to clean reference images in order to determine the fraction of dust left on each part. (C) Average spatial distribution of dust 0 min after dusting and after flies groomed for 35 min. The number of flies contributing to each heat map is displayed. (D) Dust removal across a 35-min time course. Masks define regions for counting the yellow pixels (dust) remaining on each sample. Each time point (normalized to 0-min samples) is plotted as the fraction of dust left in the defined regions and shown as the mean ± SEM; n ≥ 26 flies. Figure panel is compiled from data shown in Figure 1—figure supplement 3. (E) Representative ethogram of the five most common cleaning movements performed by an individual fly after dusting (manually scored from video recordings). All head cleaning movements are binned because eye and antennal cleaning are not easily distinguishable in the dusted state using our analysis methods (labeled whole head). (F) Latency to the first bout of head, abdomen, or wing cleaning after dusting for each of six flies annotated. (G) Transitions among different body cleaning movements, standing, and walking (across a 25-min time course, n = 6 flies). The radii of the nodes are proportional to the log of the average fraction of total cleaning bouts for each movement. Average total bouts for each movement are shown. Arrow widths represent the transition probabilities between the movements (displaying transition probabilities ≥0.05).