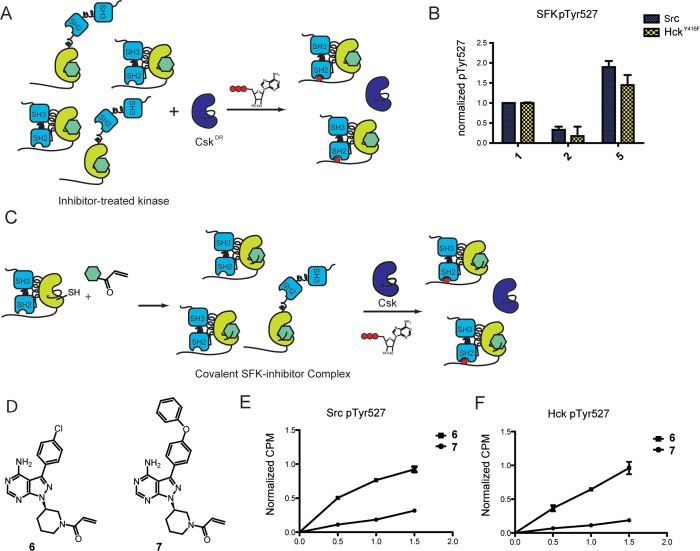
Figure 4.
SFK inhibitors can modulate C-terminal tail phosphorylation. (A) Experimental schematic of SFK Tyr527 phosphorylation. Src or HckY416F was incubated with CskDR in the presence of inhibitor. The concentration of each inhibitor is such that >85% of Src or HckY416F is bound to inhibitor and CskDR is inhibited by <13%. ATP was added to the mixture to initiate CskDR-mediated phosphorylation of the SFK–inhibitor complex. After 1 h of incubation, pTyr527 levels were determined by performing SDS-PAGE, followed by immunoblotting with an α-phospho-527 Src antibody for Src or α-pTyr antibody for HckY416F. All values are normalized to that of the SFK–1 complex. (B) Quantification of Src and HckY416F pTyr527 levels in the presence of inhibitors 1, 2, or 5. All values are normalized to that of the SFK–1 complex (mean ± SEM, n = 3). (C) Chemical genetic method for generating covalent SFK–inhibitor complexes to measure SFK Tyr527 phosphorylation. Covalent SFK–ligand complexes were generated by incubating SFKS245C with ligand 6 or 7. The resulting SFK–ligand substrate was purified and then incubated with Csk and γ32P-ATP. Phosphorylation levels were measured at 0.5, 1, and 1.5 h. (D) Structures of electrophile-containing analogues. (E) Quantification of SrcS245C pTyr527 levels in the presence of inhibitors 6 or 7 (mean ± SEM, n = 3). (F) Quantification of Hck245C pTyr527 levels in the presence of inhibitors 6 or 7 (mean ± SEM, n = 3).