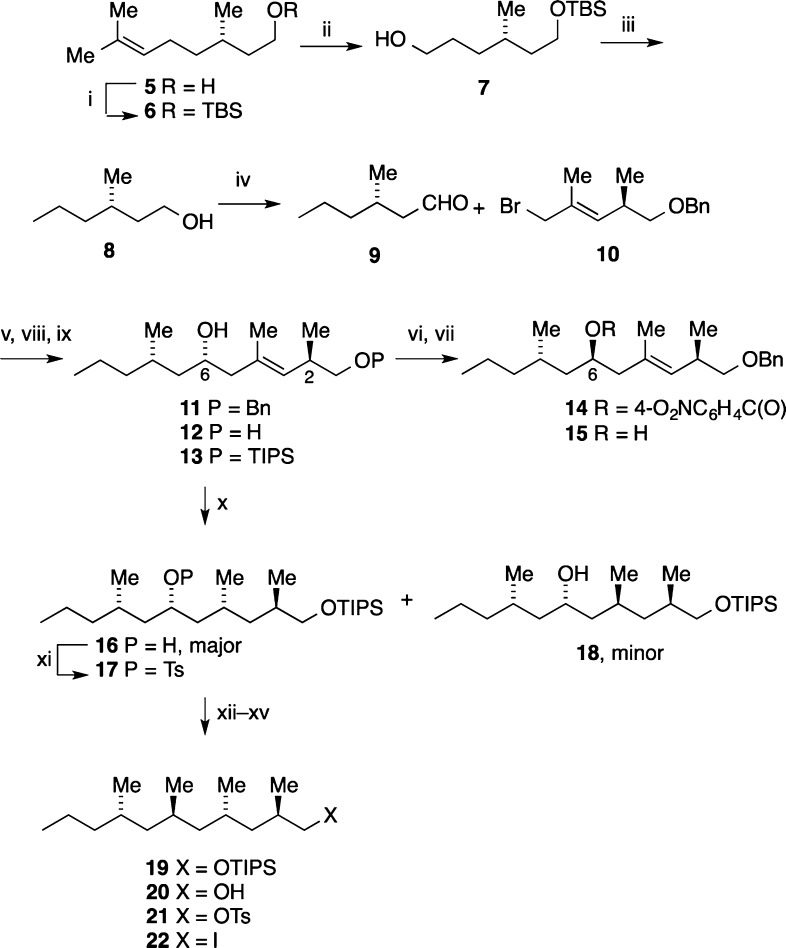
Scheme 1. Synthesis of the 1-Iodo-2,4,6,8-tetramethyl-undecane 22.
Reagents and conditions: (i) TBSCl, imid., THF, rt, 16 h (98%); (ii) O3, DCM, MeOH, −78 °C, 2 h, NaBH4, −78 °C to rt, 3 h (98%); (iii) (a) I2, Ph3P, imid., DCM, rt, 2 h; (b) KOtBu, THF, rt, 16 h; (c) 4 M aq HCl, THF, rt, 3 h; (d) H2, Pd/C, THF, rt, 16 h (60% from 7); (iv) Dess–Martin periodinane, DCM, NaHCO3, rt, 30 min; (v) BiI3, Zn powder, THF, rt, 2 h, add 9 and 10, THF, reflux, 2 h (60%); (vi) 4-O2NC6H4CO2H, Ph3P, DIAD, THF, rt, 16 h (70%); (vii) 2 N aq NaOH, THF, 50 °C, 1 h (70%); (viii) naphth., Li, THF, −25 °C, add 11, 2 h (78%); (ix) TIPSCl, imid., THF, rt, 16 h (94%); (x) [Rh(NBD)Diphos-4]BF4 (cat.), DCM, H2, 950 psi, rt, 5 h (16, 70%; 18, 16%); (xi) TsCl, DMAP, THF, rt, 16 h (93%); (xii) CuI, MeLi·LiI, 0 °C, 17, 0 °C–rt, 16 h (21%); (xiii) 4 M aq HCl, dioxane, THF, rt, 16 h (91%); (xiv) TsCl, DMAP, DCM, rt, 16 h (85%); (xv) NaI, acetone, reflux, 16 h (90%).