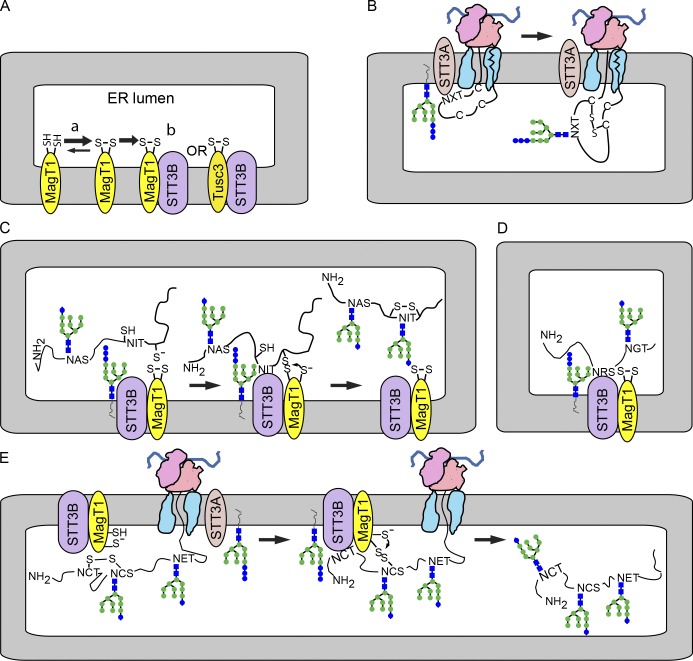
Figure 7.
MagT1-dependent glycosylation of sequons by the STT3B complex. (A) MagT1 or TUSC3, primarily in the oxidized state, assemble into the STT3B complex. (B) Cotranslational glycosylation of sequons in cysteine-rich protein domains by the STT3A complex. (C) Formation of a transient mixed disulfide between MagT1 and a glycoprotein substrate facilitates posttranslocational glycosylation of a cysteine-proximal sequon by the STT3B complex. (D) MagT1 is required for full activity of the STT3B complex even when substrates lack nearby cysteine residues. (E) The reduced form of MagT1, perhaps generated in situ, can reduce a disulfide by forming a transient mixed disulfide.