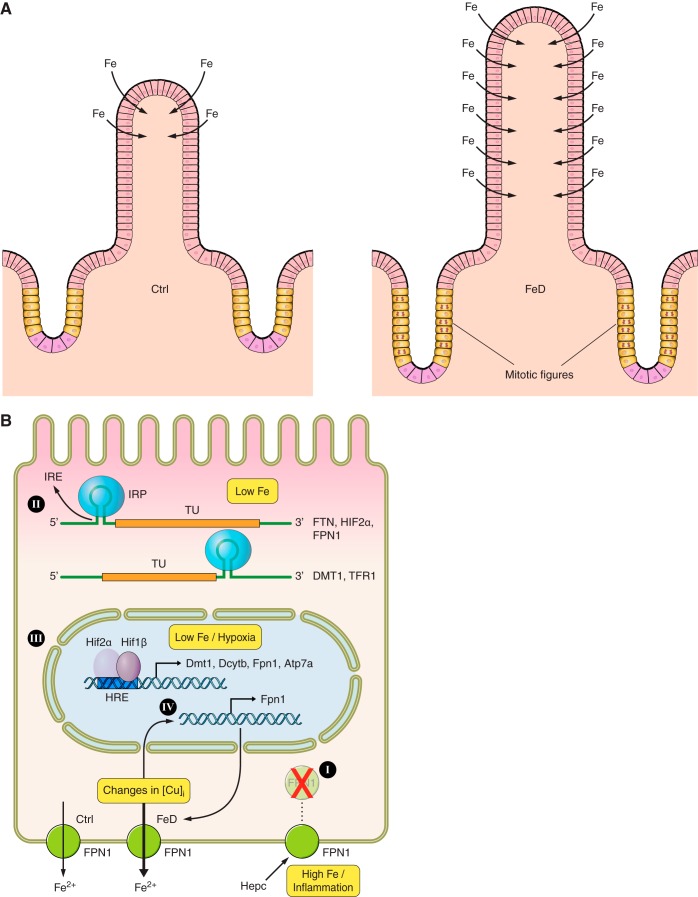
Fig. 2.
Systemic and mucosal regulation of iron absorption. During iron deficiency (FeD), morphological adaptations occur within the intestinal mucosa (A). These include increased mucosal thickness, increased villus length and width, enhanced mitosis of stem cells in the crypts (exemplified by more “mitotic figures”), and absorption of iron via enterocytes over a greater length of the villus. The net result is to maximize the capacity of the small intestine to extract iron from the diet. Additional adaptations occur within duodenal enterocytes in response to changes in intracellular metal-ion concentrations or to alterations in body iron status (B). When body iron stores are replete or during iron overload, HEPC binds to FPN1 on the BLM of enterocytes and causes its internalization and degradation, effectively blocking iron export (I). Iron absorption is also regulated locally by changes in intracellular iron levels, which alter interactions between stem-loop structures in mRNA transcripts [iron-responsive elements (IREs)] and cytosolic iron-sensing proteins [iron-regulatory proteins (IRPs)] (II). The result is that the translation of certain transcripts is blocked (e.g., ferritin [FTN], HIF2α, FPN1) and other transcripts are stabilized (e.g., DMT1, TFR1). Moreover, during iron deficiency and/or low oxygen conditions, a hypoxia-inducible trans-acting factor, hypoxia-inducible factor (HIF) 2α, is stabilized in enterocytes, promoting its dimerization with a HIF1β subunit and induction of gene transcription via interaction of the complex with a hypoxia-responsive cis-acting element (HRE) on target gene promoters (III). This results in increased iron transport via apical and BLM transport processes. Alterations in intracellular copper homeostasis, which occur during iron deficiency (FeD), have also been shown to affect iron transport in enterocytes (IV). Knockdown of a copper transporter (ATP7A), which presumably alters intracellular copper distribution and/or cuproenzyme synthesis, increases FPN1 gene transcription and enhances iron efflux. TFR1, transferrin receptor 1; Ctrl, control (normal iron status); FeD, iron-deficient; TU, transcriptional unit.