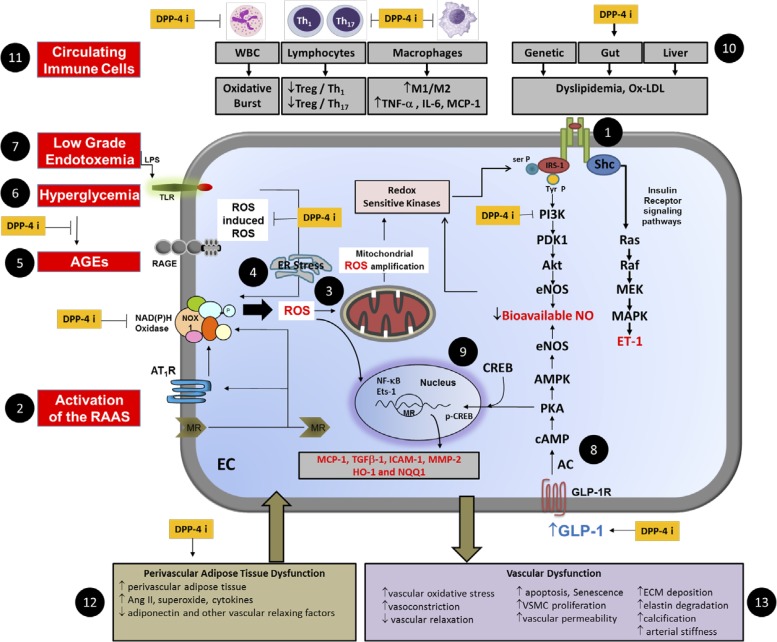
Fig. 3.
A schematic representation depicting deleterious effects of overnutrition and obesity in the vasculature and targets for DPP-4i. Numbered black circles 1 through 13 indicate key components of pathology. DPP-4i improve vascular dysfunction through GLP-1-dependent and -independent mechanisms through modulation of insulin (1) and renin-angiotensin-aldosterone system (RAAS) activation (2) leading to state of reactive oxygen species (ROS)-induced ROS and mitochondrial ROS amplification (3) and endoplasmic reticular (ER) stress (4). Advanced glycation end products (AGE) (5), hyperglycemia (6), and low-grade endotoxemia (7) collectively contribute to intracellular oxidative stress. The exacerbated state of oxidative stress induces activation of redox sensitive kinases that influence the phosphorylation state of insulin receptor substrate-1 (IRS-1) to reduce nitric oxide (NO) generation via the phosphoinositide 3-kinase (PI3K)/Akt/endothelial NO synthase (eNOS) signaling pathway while favoring signaling through the Shc Ras/MEK/MAPK to increase synthesis of endothelin-1 (ET-1). GLP-1 mediated activation of eNOS (8) and cAMP responsive element binding protein (CREB) (9) also contribute to vascular protection by DPP-4i. Dyslipidemia (10) and dysfunctional immunity (11) and dysfunctional visceral and perivascular adipose tissue (12) further contribute to vascular injury and inflammation. Thus the interaction of vascular cells, immune cells and adipose tissue causes abnormalities in vascular function and remodeling (13). DPP-4i (indicated by yellow boxes) modulate different components of pathways that could act to improve vascular function. RAGE, receptor for AGE; TLR, Toll-like receptor; Treg, regulatory T cells; Th, T-helper cells; WBC, white blood cell; MCP-1, monocyte chemoattractant protein-1; Ox-LDL, oxidized LDL; NOX, NADPH oxidase; AT1R, angiotensin type 1 receptor; MR, mineralocorticoid receptor; EC, endothelial cell; Ets-1, E26 transformation-specific-1; Ser P, serine phosphorylation; Tyr P, tyrosine phosphorylation; PDK1, 3-phosphoinositide-dependent protein kinase-1; AMPK, AMP-activated protein kinase; AC, adenylate cyclase; HO-1, hemoxygenase-1; NQQ1, quinine reductase.