Abstract
AIM
To investigate the retinal nerve fiber layer (RNFL) thickness changes in patients with obstructive sleep apnea syndrome (OSAS) for one year follow-up. To discuss the possibility of detecting tendency of glaucoma in this population by using spectral domain optical coherence tomography (3D-OCT-2000 Spectral domain).
METHODS
After polysomnographic study, all subjects (64 OSAS patients and 40 controls) underwent detailed ophthalmological examination. After these examinations, patients with glaucoma and patients who had ophthalmological and/or systemic disease were excluded from the study. Totally, 20 patients in OSAS group and five patients in controls were excluded from the study in the first examination and follow-up period. The RNFL thickness was assessed with OCT. Forty-four OSAS patients and 35 control subjects were followed up 12mo. RNFL thickness change and OSAS patients were evaluated for severity of disease by Apnea-Hypopnea Index (AHI).
RESULTS
Forty-four OSAS patients and 35 controls were enrolled in the study. Statistically significance was found between OSAS patients and controls at the 12th mo. Average RNFL thickness was found to be significantly lower in last measurements in OSAS patients when compared with first measurements and control subjects (P<0.001, 0.002, respectively). There was a statistically significant correlation among AHI, and RNFL thickness (P<0.05).
CONCLUSION
The results suggest that the patients with OSAS were related with a proportional decrease in the RNFL thickness. These patients should be followed up regularly for glaucomatous changes. Detecting more RNFL thinning in severe OSAS was important.
Keywords: obstructive sleep apnea syndrome, optical coherence tomography, retinal nerve fiber layer thickness
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a disorder characterized by recurrent airway obstructions and decreased oxygen saturation[1]. Recurrent airway obstructions during sleep cause hypoxia, hypercapnia and intrathoracic pressure changes which affect autonomic, hemodynamic, humoral and neuroendocrin regulations. These changes may influence the optic nerve head perfusion and may result in ganglion cell loss. Factors like perfusion pressure changes, systemic hypotension, night falls in the arterial blood pressure, vasospasm, increased blood viscosity and increased resistance at small vessels perfusing the optic nerve head can cause transient or permanent ischemia. As a result, optic nerve may become more sensitive to high intraocular pressure or optic nerve damage may develop even with normal intraocular pressure[2]–[4]. When nocturnal hypotension is added to the vascular disorders of OSAS, retinal nerve fiber layer (RNFL) damage and thinning may occur. The prevalence of glaucoma in the OSAS populations has been investigated in several studies and glaucoma prevalence was found to be 5.9% to 27%[2]–[5]. Additionally, patients with primary open angle glaucoma or normal-tension glaucoma constitute a high-risk population for sleep apnea syndrome[6]–[8]. RNFL thinning and characteristic visual field defects are the most common signs of glaucoma[9]. However, visual fields defects become evident only after 40% or more retinal ganglion cell axons damage, therefore early detection of RNFL thinning offers an opportunity to detect glaucoma at its earlier stages[9]–[11]. Since OSAS has been associated with glaucoma, we performed RNFL thickness measurements in patients with OSAS to determine decrease in time.
SUBJECTS AND METHODS
This comparative study consisted of 64 newly diagnosed OSAS patients and 40 controls. After polysomnographic study, patients with an apnea-hypopnea index (AHI) ≥5 were considered as OSAS and AHI <5 were included as control subjects that were matched 1:1 with OSAS patients for body mass index (BMI), sex, and age. Overnight polysomnography was performed in all subjects by a computerized system (Somnologica software). Apneas were defined as complete cessation of airflow ≥10s. Hypopneas were defined as reduction of >50% in airflow signal with a fall of ≥3% in oxygen saturation or an arousal. The Apnea-Hypopnea Index (AHI) was defined as the number of apneas and hypopneas per hour of sleep. Patients with AHI≥5 were considered as OSAS. Patients with OSAS were evaluated in three groups according to apnea-hypopnea index (AHI) scores. According to AHI scores; 5-15 score was mild, 16-30 score was moderate and over 30 score was classified as severe OSAS. The institutional board for ethics approved the study (Date: 06 Sep 2005 Decision:201) and adhered to the standards of the Declaration of Helsinki. Informed consent was obtained from every subjects participating in the study.
Patients with a history of ocular surgery, ocular trauma, anterior or posterior segment disease, glaucoma, chronic steroid use, heavy smoking, alcohol abuse, bronchial asthma, interstitial lung diseases, cerebrovascular disease, systemic hypertension, diabetes mellitus were excluded from the study.
After polysomnography all subjects underwent initial ophthalmological examination. The eye examination included the best corrected visual acuity with a recording of refractive correction, slit-lamp biomicroscopy of the anterior segment, and Goldmann applanation tonometry. Before pupillary dilation automated perimetry was made with Humphrey Field Analyzer II (Model 750, Zeiss, USA). After pupillary dilation ophthalmoscopic examination was carried out. All patients with OSAS were used continuous positive airway pressure (CPAP) device for therapy.
In all, 20 patients in OSAS group and five patients in controls were excluded from the study in the first examination and follow-up period. The reasons for exclusion from the study were as follows: one patient was found to have primary open angle glaucoma and six patients were found to have normal tension glaucoma at initial examination in OSAS group. Eighteen patients were excluded from the study as they fulfilled the above criteria and irregular follow-up.
In all, 44 patients in the OSAS group and 35 controls were included and underwent RNFL thickness measurement. Patients were evaluated three months intervals. Forty-four patients were followed up during 12mo for RNFL thickness change. Optic disc and RNFL thickness analysis were performed with 3D OCT-2000 (Topcon; Topcon Corp., Tokyo, Japan). The RNFL thickness was measured by the same ophthalmologist (MOZ). Temporal, nasal, inferior, superior quadrant peripapillary RNFL thickness were obtained from the OCT. The 3D Scan 256×256 protocol, covering a 6×6-mm2 area, was used with the 3D OCT-2000 device. Using a 3.4 mm diameter circle around the optic nerve head, circumpapillary mean global RNFL thicknesses were calculated. RNFL thickness measurements were made by two masked physicians (MOZ and EK). The average of the two measurements was taken; the interexaminer reproducibility of the choroidal thickness measurements was assessed by measuring the intraclass correlation coefficient (ICC).
The authors contributions to the study were as follows: conception and design (MOZ, EK), acquisition of data (MOZ), analysis and interpretation of data (MOZ, IT), article drafting and revising (MOZ) and final approval (MOZ).
Statistical package SPSS 17.0 for Windows was used to perform the statistical analysis. Descriptive statistics were generated for all variables. Continuous variables were demonstrated as mean±SD for normally distributed and as median (minimum-maximum) for skew distributed data. Categorical variables were given as percentages. Categorical variables were compared with chi-square test, normally distributed numeric variables were compared with paired t-test and Independent Samples “T” test. Kruskal–Wallis analysis of variance test was used to compare the RNFL measurements among the group and significant differences among the groups as determined by the analysis of variance, then Mann–Whitney U test was used to evaluate differences between groups. Correlations were tested with Pearson correlation tests. To quantify the reproducibility of manual re-measurements of the choroidal thickness in cases of alignment errors, ICC was calculated. P value < 0.05 was considered statistically significant.
RESULTS
A total of 44 OSAS patients (mean age: 52.6±8.4y) and 35 controls (mean age: 51.2±7.5y) were enrolled in the study. The AHI was calculated as the number of apneas and hypopneas per hour of sleep. It was found to be significantly increased in OSAS patients compared to the control subjects (27.4±21.7 vs 3.4±2.1, P<0.001). Mean O2 saturation (SaO2) was significantly decreased in OSAS patients compared to the control subjects (90.3±3.2 vs 94.6±2.1, P=0.005). The mean intraocular pressure (IOP) of the OSAS patients and controls showed no significant difference at baseline and last control. Baseline characteristics of the patients are shown in Table 1.
Table 1. Baseline characteristics.
| Characteristic | OSAS (n=44) | Control (n=35) | p |
| Age (mean±SD) (a) | 52.6±8.4 (34-74) | 51.2±7.5 (31-66) | 0.486 |
| M/F (%) | 27/17 (61/39%) | 23/12 (66/34%) | 0.784 |
| BMI (kg/m2) | 30.5±6.1 (20.7-49.6) | 29.6±5.1 (20.4-47.3) | 0.467 |
| AHI (mean±SD) | 27.4±21.7 (5.7-99.2) | 3.4±2.1 (0.7-4.8) | <0.001 |
| Mean SaO2 | 90.3±3.2 | 94.6±2.1 | 0.005 |
| Mean IOP (mm Hg) | 16.4±3.6 | 16.1±3.3 | 0.881 |
OSAS: Obstructive sleep apnea syndrome; BMI: Body mass index; AHI: Apnea-hypopnea index.
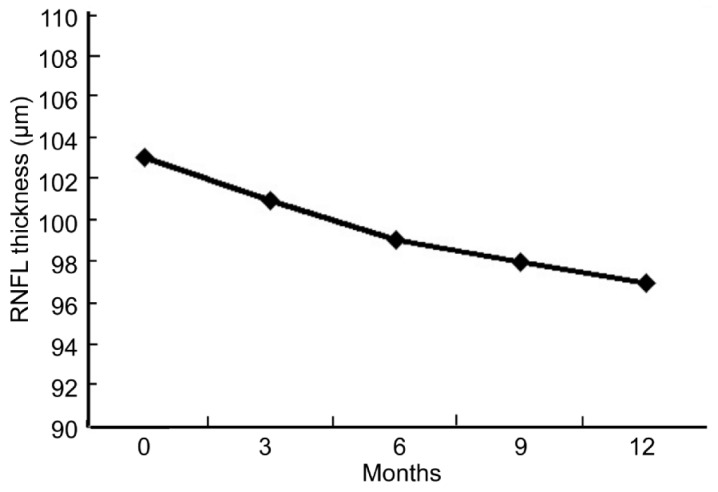
Although, baseline RNFL thickness measurements of the OSAS patients and controls showed no significant difference, statistically significance was found between OSAS patients and control subjects at last control for superior, inferior and nasal quadrants(Table 2). The average RNFL thickness which was 102.88±8.66µm (mean±SD) at first exam, decreased to 97.19±8.66 µm at last exam (P<0.001). Progressive decrease in RNFLT was observed during follow-up(Figure 1). However, average RNFL thicknesses of the control group revealed no significant difference when comparing measurements at first and last exam (P=0.877).
Table 2. Comparison of the mean RNFL thickness between baseline and at 12th mo measurements according the quadrants in OSAS patients and controls.
| RNFL thickness | OSAS patients |
Control subjects |
1P | 2P | ||
| Baseline measurements (n=44) | Last (at 12th mo) measurements (n=44) | Baseline measurements (n=35) | Last (at 12th mo) measurements (n=35) | |||
| Average | 102.88±8.66 µm | 97.19±8.66 µm | 103.55±6.64 µm | 103.90±10.37 µm | 0.002 | 0.705 |
| Temporal | 81.57±11.91 µm | 79.20±12.98 µm | 80.86±8.14 µm | 83.40±11.96 µm | 0.500 | 0.513 |
| Nasal | 88.52±14.78 µm | 81.11±13.38 µm | 89.66±8.88 µm | 89.74±15.47 µm | 0.010 | 0.690 |
| Superior | 117.18±15.22 µm | 109.31±14.86 µm | 118.20±11.60 µm | 116.83±14.26 µm | 0.026 | 0.737 |
| Inferior | 126.59±18.70 µm | 116.75±13.83 µm | 125.49±19.36 µm | 125.65±18.33 µm | 0.020 | 0.798 |
RNFL: Retinal nerve fiber layer; OSAS: Obstructive sleep apnea syndrome; 1Corresponding pairs: Baseline measurements between OSAS patients and controls; 2Corresponding pairs: Last (at 12th mo) measurements between OSAS patients and controls.
(x±s, µm)
Figure 1. The mean RNFL thickness measurements at 0, 3, 6, 9, and 12th mo.
According to AHI scores, 13 patients were in the mild group, 17 patients were in the moderate group, and 14 patients were in the severe group. There was no statistically significant difference in these three groups in terms of age, gender and BMI distribution (P>0.05). The demographic characteristics of these three groups are shown in Table 3. Mild, moderate and severe OSAS patients were compared in average RNFL thickness measurements at initially and 12th mo control and difference in first and last measurements (Table 4). There was statistically significant difference in these three groups for average RNFL thickness measurement at 12th mo (P=0.034). RNFL thickness was statistically different between mild and severe OSAS groups and moderate and severe groups (P=0.018 and P=0.035, respectively). There was no statistically significant difference between mild and moderate OSAS groups (P=0.677). There was a weak negative correlation between AHI and RNFL thickness reduction (r=-0.333, P=0.027).
Table 3. Distribution of patients according to the severity of OSAS and demographic characteristics.
| Parameters | Mild OSAS group (n=13) | Moderate OSAS group (n=17) | Severe OSAS group (n=14) | 1P |
| Age (a) (mean±SD) | 51.2±10.2 | 53.1±7.3 | 53.1±8.3 | 0.623 |
| Gender (M/F) % | 6/7 (61.5%-38.5%) | 12/5 (70.6%-29.4%) | 11/3 (64.3%-35.7%) | 0.381 |
| BMI (mean±SD) | 30.1±7.9 | 28.8±5.0 | 32.9±4.9 | 0.073 |
| AHI(mean±SD) | 8.6±3.0 | 21.5±3.6 | 52.2±21.8 | <0.001 |
OSAS: Obstructive sleep apnea syndrome; BMI: Body mass index; AHI: Apnea-hypopnea index; 1Kruskal–Wallis analysis of variance test was used.
Table 4. Comparison of RNFL thickness between mild, moderate and severe OSAS groups.
| RNFL thickness | Mild OSAS Group (n=13) | Moderate OSAS Group (n=17) | Severe OSAS Group (n=14) | 1P |
| First measurement (µm) | 101.9±9.1 | 105.3±7.8 | 100.8±9.1 | 0.215 |
| Last measurement (µm) | 99.6±9.2 | 99.1±7.7 | 92.6±8.0 | 0.034 |
| Difference in first and last measurements (µm) | 2.3±5.6 | 6.2±5.2 | 8.2±8.0 | 0.155 |
RNFL: Retinal nerve fiber layer; 1Kruskal–Wallis analysis of variance test was used.
The interexaminer ICC for the mean choroidal thickness was 0.922 (95% CI, 0.904–0.965) and ICC was greater than 0.90 for all measurement points.
DISCUSSION
Obstructive sleep apnea is a risk factor for cardiovascular and neurovascular diseases[12]. During sleep, repetitive episodes of airway occlusion, with consequent hypoxemia, hypercapnia, and changes in intrathoracic pressure elicit changes in the autonomic, haemodynamic, humoral, and neurorendocrine responses that can affect the circulation of the optic nerve with loss of ganglion cells[2]. We found that almost all of the nerve fiber layer parameters were decreased in patients with OSAS, which suggests that there is a diffuse loss of axons entering the neuroretinal rim as expected in glaucoma, especially the thickness of the superior and inferior quadrants is affected earlier in glaucoma[11].
Our study showed reduced average RNFL thickness throughout follow-up period. We observed significant reduced in RNFL thickness for superior, inferior and nasal quadrants.Therefore, it has been suggested that all measurement points of RNFLT should be evaluated. In addition, there was significant difference in RNFL thickness between OSAS groups. Especially, distinctive RNFL thickness decrease was observed in severe OSAS. To the best of our knowledge, there have not been any reports evaluating RNFL thickness changes for a long term in patients with OSAS. One year results of RNFL thickness changes in OSAS patients were given in this study. Progressive RNFL thickness thinning was observed overtime, in spite of using CPAP device for therapy. Kiekens et al[13] were described that CPAP therapy causes an additional IOP increase, especially at night. Regular screening of visual fields and the optic disc is warranted for all patients with OSAS, especially those treated with CPAP. These findings may explain RNFL thining overtime, such as our study.
In glaucoma, RNFL thickness decreases progressively. This thinning can be present in eyes of patients with glaucoma before detectable changes occur in the visual field[9],[10]. If a decrease in the RNFL can be reliably detected, the clinician may be alerted to the risk for developing glaucoma. Kargi et al[14] were reported RNFL thickness in patients with OSAS firstly. Kargi et al[14] demonstrated that sleep apnea syndrome is correlated with a proportional decrease in the RNFL thickness. Afterwards, a few studies have investigated RNFL parameters. These studies were demonstrated RNFL thinning in OSAS patients[15]–[18]. Moreover these findings, we followed up OSAS patients and progressive thinning of RNFL thickness was observed in one year follow-up period.
In our study, we detected a moderate correlation between AHI and measurements of RNFL thickness and progressive thinning of RNFL thickness in spite of using CPAP device for treatment. Especially more RNFL thickness thinning was observed in severe OSAS. It may be suggested that RNLF thickness measurement, and regular follow-up are important for OSAS patients.
Hayreh et al[19] and Renard et al[20] reported that nocturnal hypotension may play a role in the pathogenesis of normal tension glaucoma. Glaucomatous optic nerve damage has lately been considered to be the result of vascular and other pathogenic mechanisms in addition to elevated intraocular pressure[6],[21]. In patients with OSAS, the sleeping state is associated with a reduction in the ventilatory drive caused by hypoxia and hypercapnia.
This causes a decrease in PO2 and an increase in PCO2[1]. Hypoxaemia causing increased vascular resistance via increased levels of the vasoconstrictor endothelin production and decreased the levels of vasodilator nitric oxide production. The endothelial cells also produce nitric oxide, a vasodilator[22],[23]. In patients with OSAS, the endothelium-mediated vasoconstrictor and vasodilator balance is markedly impaired. Vasodilator response markedly decreased. Optic disc damage may be caused by a loss of ganglion cells caused in some way by the hypoxia secondary to this OSAS-induced imbalance between nitric oxide and endothelin[22],[23]. Increased vascular resistance in patients with OSAS may impair perfusion and oxygenation of the optic nerve head. With the increasing severity of OSAS, mean oxygen saturation values are reduced and relative hypoxia occurs. We found that, as the disease becomes more severe, RNFL thinning is proportionally more prominent. This supports the correlation founded between hypoxia and RNFL thinning in our study.
Nocturnal vascular changes caused by OSAS may be the cause of RNFL thinning and glaucomatous optic nerve damage. Hypoxia indirectly increased intracranial pressure during sleep and decreased cerebral perfusion pressure may disturb blood supply of the optic nerve in patients with OSAS[6],[24]. Vascular disturbances may result with diffuse loss or localized defects of the RNFL before initiation of glaucoma[25].Our major limitation is the limited number of cases. However, it is very difficult to obtain OCT images during follow-up period.
In conclusion, in this study we found that patients with OSAS have a tendency to glaucoma. These patients have to be followed regularly for glaucoma development and progression. It may be cautious for clinicians to consider the possibility of glaucoma in OSAS patients and vice versa. OSAS patients should be followed up regularly for glaucomatous changes though treated with CPAP. Further studies should be done on the pathogenesis of RNFL thinning in this group of patients.
Acknowledgments
Conflicts of Interest: Zengin MO, None; Tuncer I, None; Karahan E, None.
REFERENCES
- 1.Phillipson EA. Sleep disorders. In: Murray JF, Nadel JA, editors. Textbook of Respiratory Medicine. WB Saunders: Philadelphia; 1994. pp. 2301–2304. [Google Scholar]
- 2.Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C, Mathis J. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999;106(5):1009–1012. doi: 10.1016/S0161-6420(99)00525-4. [DOI] [PubMed] [Google Scholar]
- 3.Khandgave TP, Puthran N, Ingole AB, Nicholson AD. The assessment of sleep apnoea as a risk factor in glaucoma. J Clin Diagn Res. 2013;7(7):1391–1393. doi: 10.7860/JCDR/2013/5383.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendel RE, Kaplan J, Heckman M, Fredrickson PA, Lin SC. Prevalence of glaucoma in patients with obstructive sleep apnoea: across-sectional case-series. Eye(Lord) 2008;22(9):1105–1109. doi: 10.1038/sj.eye.6702846. [DOI] [PubMed] [Google Scholar]
- 5.Lin CC, Hu CC, Ho JD, Chiu HW, Lin HC. Obstructive sleep apnea and increased risk of glaucoma: a population-based matched-cohort study. Ophthalmology. 2013;120(8):1559–1564. doi: 10.1016/j.ophtha.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Faridi O, Park SC, Liebmann JM, Ritch R. Glaucoma and obstructive sleep apnoea syndrome. Clin Experiment Ophthalmol. 2012;40(4):408–419. doi: 10.1111/j.1442-9071.2012.02768.x. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez-Díaz E, Perez-Rico C, de Atauri MJ, Mencía-Gutierrez E, Blanco R. Evaluation of the visual function in obstructive sleep apnea syndrome patients and normal-tension glaucoma by means of the multifocal visual evoked potentials. Graefes Arch Clin Exp Ophthalmol. 2012;250(11):1681–1688. doi: 10.1007/s00417-012-1982-z. [DOI] [PubMed] [Google Scholar]
- 8.Lin PW, Friedman M, Lin HC, Chang HW, Wilson M, Lin MC. Normal tension glaucoma in patients with obstructive sleep apnea/hypopnea syndrome. J Glaucoma. 2011;20(9):553–558. doi: 10.1097/IJG.0b013e3181f3eb81. [DOI] [PubMed] [Google Scholar]
- 9.Kremmer S, Ayertey HD, Selbach JM, Steuhl KP. Scanning laser polarimetry, retinal nerve fiber layer photography, and perimetry in the diagnosis of glaucomatous nerve fiber defects. Graefes Arch Clin Exp Ophthalmol. 2000;238(11):922–926. doi: 10.1007/s004170000196. [DOI] [PubMed] [Google Scholar]
- 10.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 11.Leung CK, Lam S, Weinreb RN, Liu S, Ye C, Liu L, He J, Lai GW, Li T, Lam DS. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: analysis of the retinal nerve fiber layer map for glaucoma detection. Ophthalmology. 2010;117(9):1684–1691. doi: 10.1016/j.ophtha.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Lanfranchi P, Somers VA. Obstructive sleep apnea and vascular disease. Respir Res. 2001;22(6):315–319. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiekens S, Veva De Groot, Coeckelbergh T, Tassignon MJ, van de Heyning P, Wilfried De Backer, Verbraecken J. Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea. Invest Ophthalmol Vis Sci. 2008;49(3):934–940. doi: 10.1167/iovs.06-1418. [DOI] [PubMed] [Google Scholar]
- 14.Kargi SH, Altin R, Koksal M, Kart L, Cinar F, Ugurbas SH, Ayoglu F. Retinal nerve fibre layer measurements are reduced in patients with obstructive sleep apnoea syndrome. Eye(Lord) 2005;19(5):575–579. doi: 10.1038/sj.eye.6701582. [DOI] [PubMed] [Google Scholar]
- 15.Calvo P, Ferrández B, Ferreras A, Marín JM . Retinal nerve fiber layer thickness alterations in patients with obstructive sleep apnea. Arch Soc Esp Oftalmol. 2012;87(1):1–2. doi: 10.1016/j.oftal.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Moghimi S, Ahmadraji A, Sotoodeh H, Sadeghniat K, Maghsoudipour M, Fakhraie G, Latifi G, Nassiri N, Giaconi JA. Retinal nerve fiber thickness is reduced in sleep apnea syndrome. Sleep Med. 2013;14(1):53–57. doi: 10.1016/j.sleep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Lin PW, Friedman M, Lin HC, Chang HW, Pulver TM, Chin CH. Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea/hypopnea syndrome. Graefes Arch Clin Exp Ophthalmol. 2011;249(4):585–593. doi: 10.1007/s00417-010-1544-1. [DOI] [PubMed] [Google Scholar]
- 18.Nowak MS, Jurowski P, Gos R, Prost ME, Smigielski J. Pulsatile ocular blood flow in subjects with sleep apnoea syndrome. Arch Med Sci. 2011;7(2):332–336. doi: 10.5114/aoms.2011.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117(5):603–624. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 20.Renard E, Palombi K, Gronfier C, Pepin JL, Noel C, Chiquet C, Romanet JP. Twenty-four hour (Nyctohemeral) rhythm of intraocular pressure and ocular perfusion pressure in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2010;51(2):882–889. doi: 10.1167/iovs.09-3668. [DOI] [PubMed] [Google Scholar]
- 21.Hayreh SS. The role of age and cardiovascular disease in glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43(Suppl 1):S27–42. doi: 10.1016/s0039-6257(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 22.Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stiefel P, Sánchez-Armengol MA, Villar J, Vallejo-Vaz A, Moreno-Luna R, Capote F. Obstructive sleep apnea syndrome, vascular pathology, endothelial function and endothelial cells and circulating microparticles. Arch Med Res. 2013;44(6):409–414. doi: 10.1016/j.arcmed.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Stein JD, Kim DS, Mundy KM, Talwar N, Nan B, Chervin RD, Musch DC. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011;152(6):989–998. doi: 10.1016/j.ajo.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayreh SS, Jonas JB. Appearance of the optic disk and retinal nerve fibre layer in atherosclerosis and arterial hypertension: an experimental study in rhesus monkeys. Am J Ophthalmol. 2000;130(1):91–96. doi: 10.1016/s0002-9394(00)00387-1. [DOI] [PubMed] [Google Scholar]