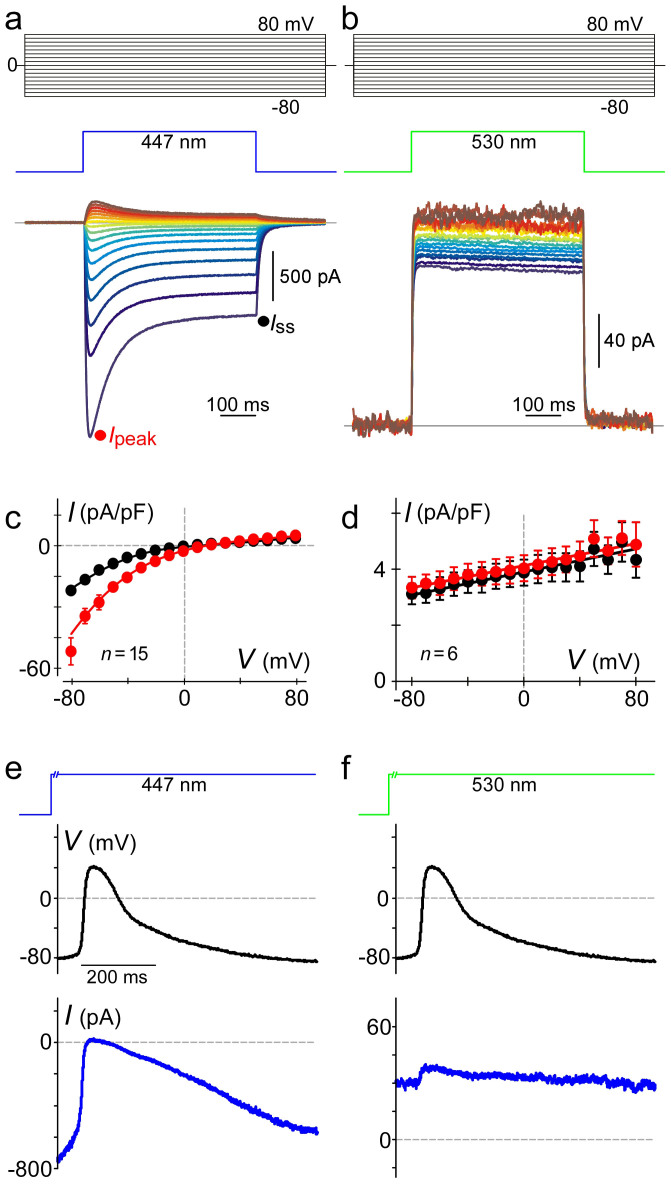
Figure 1. Biophysical properties of optogenetic constructs and their suitability for altering excitability during action potentials.
ChR2 (a, c) and eNpHR3.0 (b, d) were initially characterized at various constant levels of voltage, using whole-cell patch clamp in HEK293 cells. (a) ChR2 supports light-gated, voltage-dependent currents with a reversal potential of ~17 mV according to the curve fit of the I-V relation in (c). Partial inactivation of currents is clearly present. (b) eNpHR3.0 is light-gated, but only weakly voltage-dependent. (c) ChR2 population data for peak (red) and steady-state (black) current levels. n refers to number of cells; bars show sem. (d) eNpHR3.0 population data for peak (red) and steady-state (black) currents. n refers to number of cells; bars show sem. Panels (e) and (f) examine optogenetic currents during voltage-clamp with cardiac action-potential waveforms gauged from neonatal rat ventricular myocytes probed with PGH1 as the voltage-sensitive dye. (e) ChR2 currents during APWs are large and enhanced by repolarization. Current amplitude between the maximum during the trace onset at −80 mV, and the minimum near the peak of the APW, was 427 ± 159 pA (mean ± sem, n = 5 cells). (f) eNpHR3.0 currents during APWs are smaller but largely independent of voltage. Current amplitude at the trace onset 32 ± 0.4 pA (mean ± sem, n = 12 cells). All blue illumination at 7.2 mW/mm2. All green illumination at 1.4 mW/mm2. Axis breaks (500 ms) in light-power schematics atop (e) and (f), ensure that channels had reached near steady-state (in)activation with respect to light, prior to trace onset.