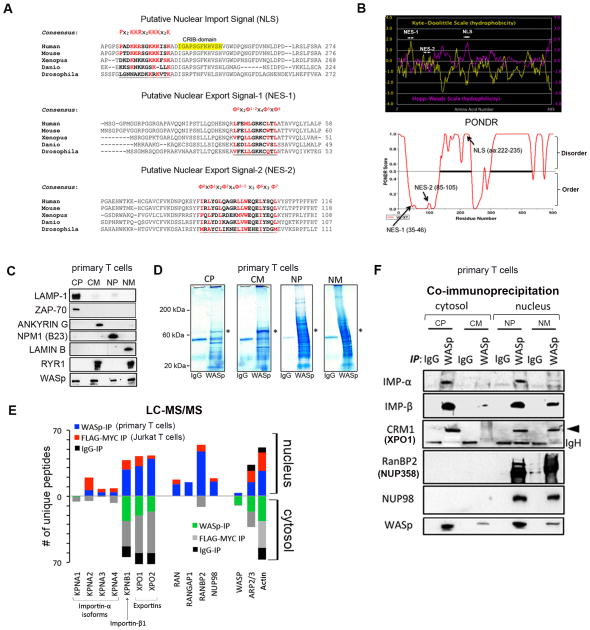
Figure 1. WASp nucleocytosolic transport sequences and partner proteins.
(A) Putative NLS- and NES-motifs (underlined) of human WASp aligned with WASp-like proteins of other species. The evolutionary conserved core amino acids known to impart NLS- or NES-specific function are indicated in red. The “ϕ” in the NESs denote hydrophobic amino acid. (B) The hydropathy profiles of the putative NLS and NES-motifs of human WASp computed from the standard algorithms of Kyte-Doolittle (in yellow) and Hopp-Woods (in pink). PONDR plot of WASp, which predicts intrinsically-disordered regions (IDRs) in WASp is also shown. A score > 0.5 predicts disordered domains and <0.5 ordered domains. (C) Subcellular fractionation of T cell compartments. The purity of the indicated fractions (CP, CM, NP, NM) isolated from TH1-skewed human primary cells probed with the indicated antibodies. CP, cytoplasm depleted of total cellular membranes; CM, purified cellular membranes; NP, nucleoplasm depleted of total cellular membranes; NM, purified nuclear membranes. (D) Coumassie staining of proteins IP’ed with anti-WASp or control IgG antibody from the indicated fractions of primary TH1-skewed cells. A ~65kDa band corresponding to WASp is indicated by an asterisk. Excised bands were analyzed by MS. (E) The actual number of polypeptides of WASp-associated, nucleocytoplasmic transport proteins in the cell fractions are shown for cytosolic and nuclear WASp-complexes immunoprecipitated (IP) with anti-WASp, -FLAG/MYC (2-step immunoaffinity purification), or -IgG Abs. (F) Validation of MS-generated WASp-transport proteome by Co-IP. Protein complexes IP’ed by anti-WASp or control Ig antibody from TH1-skewed cells were resolved by sequential Western blotting the same gel using indicated antibodies. Loaded IP material is ~10% of input.