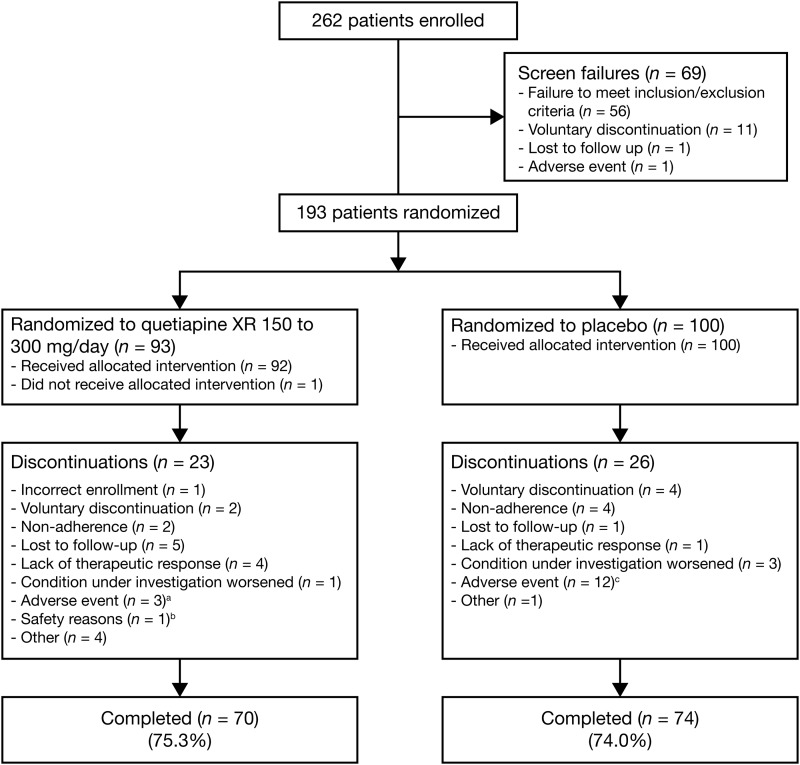
FIG. 1.
Disposition of youth with bipolar depression in the quetiapine (150–300 mg/day) and placebo groups. aDiscontinued because of: headache, sedation, and increased fatigue (n=1); somnolence (n=1); and a serious adverse event of agitation (n=1). bAssessed by investigator; no further information available. cDiscontinued because of: irritability (n=3; one of whom also reported headache); somnolence (n=2); sedation, neutropenia, and neutrophil count decreased (n=1, each); and serious adverse events of aggression, social stay hospitalization, exacerbation of bipolar I symptoms, and exacerbation of depressive symptoms (n=1, each).