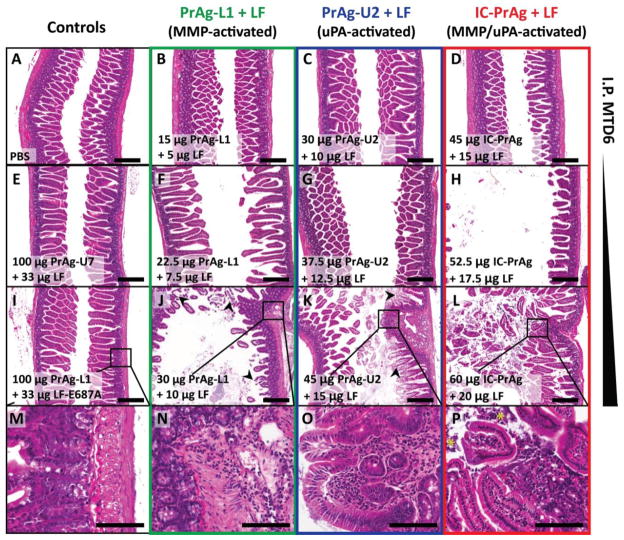
Figure 1. GI toxicity is dose-limiting when C57BL/6J mice are treated with six I.P. doses of MMP-, uPA- or dual MMP/uPA-activated anthrax lethal toxins.
Representative H&E sections of small intestine depicting the dose-dependent progression of GI toxicity observed when PrAg-L1 + LF (B,F,J,N) PrAg-U2 + LF (C,G,K,O) or IC-PrAg + LF (D,H,L,P) are administered intraperitoneally. At the MTD6 for each toxin, no GI pathology was present at the gross or microscopic level in 29/30 experimental mice (B–D). At this dose, note the similarity in appearance to control-treated mice receiving 6 I.P. doses of either PBS (A), uncleavable anthrax PrAg paired with LF, 100 μg PrAg-U7 + 33 μg LF (E), or MMP-activated PrAg paired with enzymatically-inactive cytotoxin, 100 μg PrAg-L1 + 33 μg LF-E687A (I). As doses were increased above the MTD6, GI toxicity initially presented as mild small intestinal dilation (F–H) which progressed in severity to involve GI inflammation, regions of villous necrosis, denuded and ulcerated GI epithelium and/or grossly visible GI hemorrhage (J–L, arrowheads depict necrotic villi). (M–P) Higher magnification images showing inflammation in the lamina propria of toxin-treated mice, but not controls. (P) * indicates inflammatory cells that have invaded into the lumen of the small intestine. Scale bars are 300 μm A–L; 100 μm M–P.