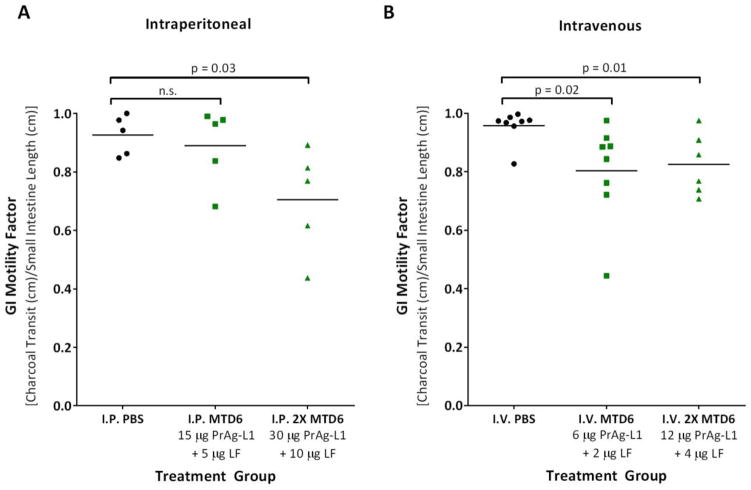
Figure 2. I.P. or I.V. treatment with MMP-activated PrAg-L1 + LF caused reduced GI motility.
3 doses of PBS (black) or PrAg-L1 + LF (green) were administered either intraperitoneally (A) or intravenously (B). At both the I.P. and I.V. MTD6s, no physical evidence of GI abnormalities was anticipated, while at doses of 2XMTD6 GI histopathology was expected. P-values were determined using a Student’s T-test, two-tailed.