Abstract
Objective
To examine the ultrastructural characteristics of extracellular matrix and mature collagen fibrils in uterine leiomyomas and compare them with those in adjacent normal myometrium.
Design
Analysis of paired leiomyoma-myometrium in surgical specimens.
Setting
Research center and tertiary care center.
Subject(s)
Women undergoing medically indicated hysterectomy for symptomatic uterine leiomyomas.
Intervention(s)
None.
Main Outcome Measure(s)
Appearance and spatial orientation of the collagen fibrils in leiomyomas compared with myometrium.
Result(s)
Observation of specimens at ×12,500 magnification indicated that collagen fibrils were more abundant, loosely packed, and arrayed in a nonparallel manner in leiomyomas compared with myometrium. Random areas were examined at ×6,500 to ×64,000 magnification and revealed collagen fibrils of equal diameter in both leiomyomas and myometrium. However, an ordered and regular barbed appearance was present in collagen fibrils from myometrium but was lacking in leiomyomas.
Conclusion(s)
Leiomyomas contain an abnormal collagen fibril structure and orientation, which suggests that the well-regulated fibril formation in myometrium is altered in leiomyomas. Alterations in collagen genes may play a role in the pathogenesis of leiomyomas.
Keywords: Leiomyomas, collagen, collagen fibrillogenesis, electron microscopy
Leiomyomas are the most common benign tumors of the uterus and are the leading cause of hysterectomy in the United States. Leiomyomas are monoclonal tumors, thus each one develops independently from other leiomyomas in the uterus. These tumors enlarge and may cause bleeding, pain, and in some instances infertility in reproductive-age women.
Genomic microarrays have offered novel insights into the pathogenesis of these highly prevalent tumors (1-5) and have demonstrated both over- and underexpression of numerous genes not previously associated with leiomyomas. Using an Affymetrix U133 platform, (Affymetrix, Santa Clara, CA) our laboratory has noted that genes associated with formation of the extracellular matrix were differentially expressed in the array results (6). Specifically, overexpression of transforming growth factor (TGF) beta-3 and TGF-RII and altered levels of extracellular proteins such as dermatopontin and versican were consistent with the conclusion that leiomyomas may possess an abnormal extracellular matrix (6). Several of these genes were collagen subtypes and collagen-related genes.
Collagens are abundant proteins encoded by at least 30 genes and make up one-quarter of the mammalian protein mass. Specific collagen types have the same amino acid sequence and molecular structure in almost all animal species and in human individuals. Fibril-forming collagens are synthesized as procollagen molecules that are secreted into the extracellular matrix, where these propeptides undergo processing and self-assembly. Once mature collagen is formed it is rarely, if ever, altered (7). Previous published studies have reported increased procollagen mRNA as well as some changes in collagen at the protein level in leiomyomas (8-10). Decreased cell size, increased cell density, and larger collagen fibrils in GnRH agonist–treated leiomyomas compared with untreated leiomyomas have been reported (11). However, detailed characteristics of these fibrils have not been described. Several reports of electron microscope studies have focused on the smooth muscle cells of leiomyomas. These studies, however, did not comment on the extracellular matrix other than to note its collagenous nature (12-14).
Despite the important role of collagen in the formation of leiomyomas, no publications have reported ultrastructure studies of the extracellular matrix of these tumors nor have the characteristics of their mature fully formed collagen been described. To study the structure and orientation of mature collagen fibrils, we conducted extensive light and electron microscope observations of the extracellular matrix in leiomyomas and anatomically adjacent myometrium. In addition, the cellular components of leiomyomas and myometrium were also carefully observed at the light and electron microscopic levels.
MATERIALS AND METHODS
Subjects
A total of five leiomyoma-myometrium paired tissue samples were immediately obtained at hysterectomy at the National Naval Medical Center with approval by that institution’s Institutional Review Board as well as the approval of the National Institutes of Health Institutional Review Board and each individual subject’s informed consent. All subjects underwent medically indicated hysterectomy for symptomatic leiomyomas. The ages of the subjects ranged from 34 to 58 years; three subjects were African-American, and two were Caucasian. One subject was menopausal, one was in the follicular phase of the cycle, one was in the luteal phase, and two had a history of menometrorrhagia in addition to leiomyomas. At the time of the evaluation of the electron micrographs, the investigators were blinded to the subject’s clinical history. A review of the clinical chart indicated that no subject had received hormone therapy. The number of fibroids per subject ranged from three to six, with a size range of 0.25 × 0.25 × 0.25 cm to 8 × 5.3 × 5.5 cm.
Tissue Collection
Samples were obtained from all leiomyomas in each subject in an area that was typically white and nonnecrotic and not infarcted. All samples were taken at a distance from the leiomyoma capsule. The myometrium used as control tissue was typically obtained 1 cm from the leiomyoma to ensure that nonleiomyoma tissue was sampled and that myometrium compressed by the leiomyoma was not obtained. Initial sample sizes obtained from the leiomyomas ranged from 3 mm3 to 1 cm3. For electron microscopy, the samples were immediately selected and fixed in 3% glutaraldehyde. Specimens were immediately placed into phosphate-buffered formalin for the light microscope studies. Tissue from all five subjects was then processed for electron and light microscopy.
Tissue Preparation
For electron microscopy, the tissue was then postfixed in 1% osmium tetraoxide followed by dehydration in graded alcohols and propylene oxide and then fixed in Epox 812 (Electronmicroscopy Sciences, Fort Washington, PA). Sections were cut on an ultramicrotome, placed on 200-mesh grids, and stained with uranyl acetate and lead citrate. A total of 186 mesh grids were prepared.
For light microscopy, paraffin sections were stained by Masson’s trichrome method. Briefly, the tissue was deparaffinized and hydrated using distilled water and then placed in Bouin’s solution for 1 hour at 56°C. They were then cooled and cleared in running water, placed in Wiegert’s iron hematoxylin solution for 10 minutes, followed by a 10-minute wash in running water, and then rinsed in distilled water. The slides were fixed in Biebrich scarlet-acid fuchsin solution for 4 minutes, rinsed and placed in phosphomolybdic-phosphotungstic acid solution for 15 minutes, and then placed in aniline blue solution for 5 minutes, followed by glacial acetic acid for 5 minutes.
Electron Microscopy
Two of the authors (PCL and TB) examined the grids under a Philips Electron Microscope (Philips Electronics, Eindhoven, Netherlands). In addition, one other study investigator also observed the grids. Each grid was observed at ×12,500 magnification followed by examination of random areas at ×6,500 to ×64,000 magnification. On some occasions the magnification was increased to ×180,000.
Light Microscopy
The multiple tissue samples stained with Masson’s trichrome were examined using a Leica microscope (Leica Microsystems, Wetzler, Germany) with both low- and high-powered lenses.
RESULTS
Light microscopy of leiomyomas and paired myometrium using stains for collagen revealed collagen to be abundant in the leiomyoma tissue, while the myometrium had sparse, well-aligned collagen bundles adjacent to smooth muscle cells consistent with our previous report (6). We did not observe leukocytes or red blood cells or blood vessels in the specimens.
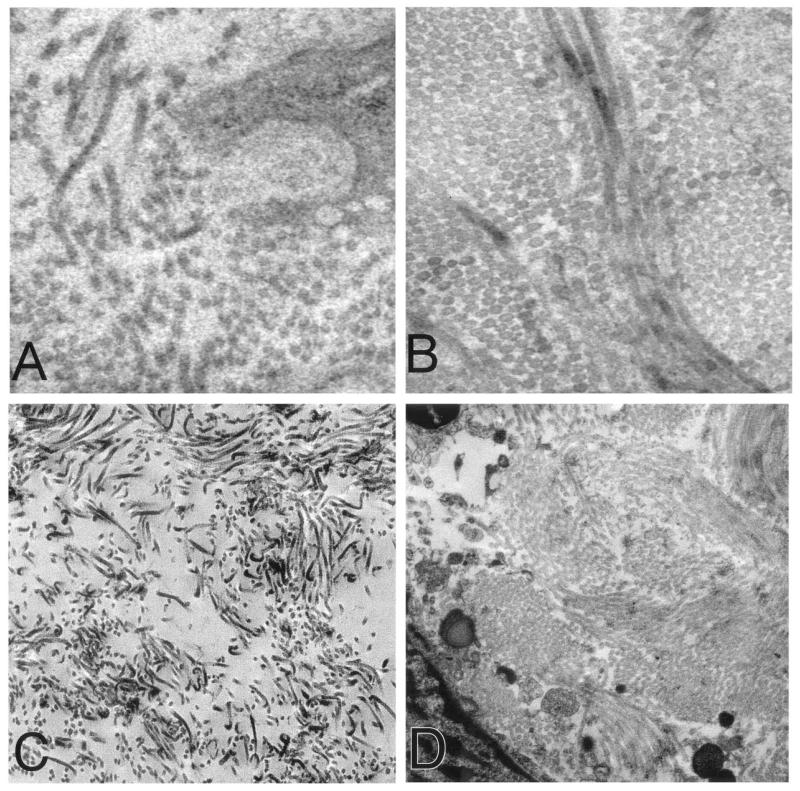
The electron microscope findings demonstrated widely dispersed and short collagen fibrils in leiomyomas compared with adjacent myometrium (Fig. 1A-1D). Consistent with the presence of an abnormal extracellular matrix, collagen fibrils were arrayed in a nonparallel manner in all leiomyomas studied, while those of the myometrium were well packed and parallel to each other. There were no differences in the fibrils in premenopausal compared with postmenopausal women. The fibrils observed in leiomyoma and myometrial tissues were of similar diameter. We found no instances where the thicknesses of the fibrils were either greater or smaller in leiomyomas compared with myometrium.
FIGURE 1.
(A), Electron micrograph (×31,000) of leiomyomas demonstrating short, randomly aligned, and widely dispersed collagen fibrils. Tissue sample was obtained from a region near the capsule of the leiomyoma. The subserosal midcavity leiomyoma measured 0.3 × 0.3 × 0.3 cm. This 58-year-old subject had a total of six leiomyomas. She received no hormonal therapy. (B), Electron micrograph (×31,000) of paired myometrium. Collagen fibrils of the adjacent myometrium were closely packed and well aligned in a parallel manner. The myometrium was obtained 0.5 cm from the edge of the leiomyoma. (C), Leiomyoma sample from subject 2 also showing loosely packed collagen fibrils and a random orientation (×12,500). This sample was subserosal and measured 3.8 × 3.5 × 2.8 cm. This 46-year-old subject had a total of four leiomyomas and did not receive hormonal therapy. (D), Electron micrograph (×12,500) of myometrium adjacent to the leiomyoma in C revealing well-packed collagen fibrils.
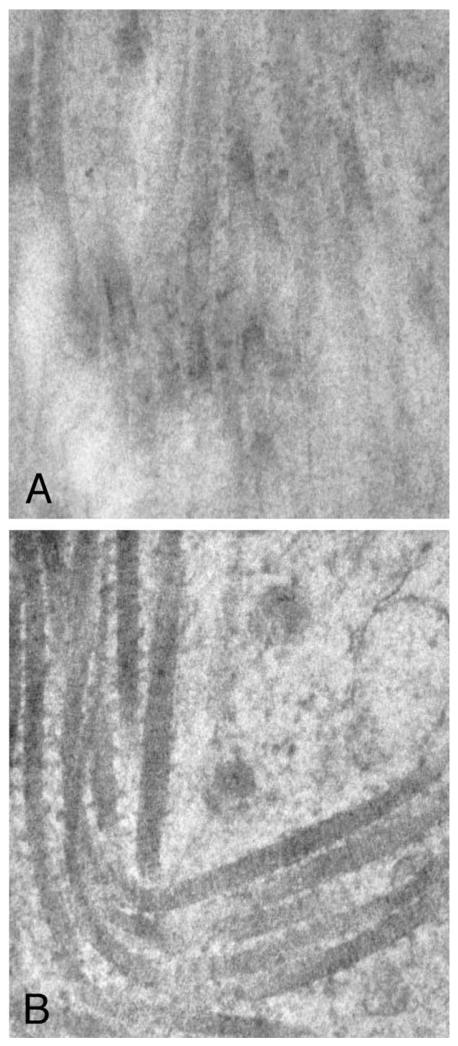
Perhaps the most intriguing finding was that collagen fibrils in the myometrium had a regular, ordered, and barbed appearance at higher magnification (Fig. 2A-2B). Despite extensive observations, we did not observe this same ordered barbed appearance in leiomyomas (Fig. 2A-2B). The collagen was observed to be completely extracellular in location. We did not note any evidence of intracellular processing as described by other investigators (15), despite extensive study. We did find that the cellular wall membranes in the leiomyomas were indistinct in a number of instances in contrast with the cells of the myometrium (data not shown). The smooth muscle cells and fibroblasts in the myometrium and the leiomyomas (data not shown) did not appear to be dissimilar, a finding reported by other investigators (12).
FIGURE 2.
A, Electron micrograph of a leiomyoma (×64,000). Note the disorganization of the collagen fibrils. There was no observed evidence of a barbed appearance of fibrils in the leiomyoma tissue studied. This sample was taken from a 0.3 × 0.3 × 0.3 cm fibroid. Subject 4 was 35 years old and had three leiomyomas. B, At the same magnification, the collagen fibrils in the myometrium have a clear and distinct barbed appearance, suggesting heterotypic fibrils. The myometrium was obtained 2.5 cm from the edge of the leiomyoma. The subject had been taking FeSO4 for uterine bleeding.
DISCUSSION
This study is the first to demonstrate that the abundant mature collagen in leiomyomas is abnormal in structure and orientation in contrast with that of myometrium. This observation expands upon our previous work (6) and suggests that these benign tumors grow by a process involving the changing transcription and expression of extracellular matrix genes. Similar abnormalities in collagen fibrils and their spatial orientation were found in all leiomyomas studied regardless of the size of tumor or subject menstrual status. Despite abnormal orientation, the diameters of the collagen fibrils did not appear to be different in the leiomyomas compared with the myometrium.
Collagens are secreted into the extracellular space by fibroblasts, smooth muscle cells, and chondrocytes, as well as other connective tissue cells, and undergo processing and assembly into fibrils in the extracellular matrix (7, 16, 17). Fibrillar collagens (types I, II, III, IV, V) are the most abundant collagens and function as structural proteins, but they have roles in developmental biology as well (18). Mature cross-linked fibrillar collagen is a large protein molecule composed of a triple helix of alpha chains of proteins each coiled into a left-hand helix. The three chains are then wrapped around each other into a right-hand helix. Collagen gene mutations are responsible for diseases such as osteogenesis imperfecta and Ehlers–Danlos and Alport syndromes (7).
Messenger RNA (mRNA) overexpression for both procollagen type I and type III has been reported in leiomyomas, however, the investigators did not comment on the presence of mature cross-linked collagen (8). Data from our previous studies reveal that a large number of extracellular matrix proteins, collagen-processing enzymes, and collagen genes were differently expressed in leiomyomas compared with adjacent myometrium (1, 6, 19). Such alterations in extracellular genes suggest significant changes in collagen gene expression in leiomyomas.
The complex biosynthesis of fibrillar collagen is well documented (7, 15, 17). Intact procollagen molecules are secreted into the extracellular compartment and then form fibrillar complexes of fibers or mesh works as their final structure depending on the type of collagen. In the extracellular space, the procollagen molecule is cleaved by specific proteinases, removing N-propeptides and C-propeptides to form the mature protein (7, 17). The collagen self-assembles into fibrils and then forms complex cross links from lysine and hydroxylysine due to the action of the enzyme lysyl oxidase. Mature cross-linked fibrillar collagens are extremely rigid and not easily degraded by tissue enzymes, including matrix metalloproteinases (16). Furthermore, proteoglycans, such as decorin, versican, and dermatopontin, are all important in the process of fibril formation (20-22). Over- or underexpression of various collagen genes causes severe alterations in the collagen processing, resulting in poorly formed fibrils as noted in keloids (6). In leiomyomas, the poorly formed collagen fibrils are abnormally oriented relative to each other and could contribute to the extremely hard fibrotic tissue of these tumors.
In addition to the effect of procollagen processing and glycosylation of collagen molecules and collagen-proteoglycan interactions on the formation of fibrils, it has been shown that the interaction of two or more different types of fibrillar collagen chains may interact and result in the formation of heterotypic fibrils (23-26). We observed collagen fibrils with an ordered and regular barbed appearance in the myometrium (Fig. 2B), which resembles that observed in chick corneas (26). The appearance of these fibrils in electron micrographs along with amino acid sequence data suggests that type V collagen molecules may occur within a heterotypic type I + V collagen fibril. These heterotypic fibrils regulate their fibril structure and diameter.
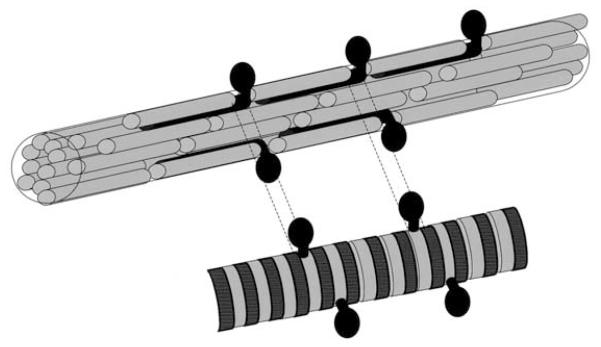
In this model, type V molecules are aligned so that their triple helical domains are within the interior of the fibril with the NH2-terminal domains extending through a gap in the fibril surface to give the barbed appearance in electron micrographs (see Fig. 3). The electron microscope findings are consistent with the presence of heterotypic fibrils in normal myometrium, but not in leiomyomas. This observation suggests that the random orientation and dispersion of the fibrils in leiomyomas may be due in part to their lack of heterotypic fibrils. Furthermore, the presence of heterotypic fibrils in myometrium would assist in regular fibril formation in normal uterine tissue. Additional studies are indicated to explore this aspect of collagen processing in leiomyomas.
FIGURE 3.
Schematic representation of collagen fibril structure. The ordered, barbed appearance noted in myometrial collagen suggests the presence of heterotypic fibrils (black bars) with the protrusion of the N-terminal region through the opening in the quarter-staggered array between ordered fibrils (gray bars). The lower fibril represents the electron micrographic fibril appearance.
This study indicates that the collagen fibril orientation and alignment observed in leiomyomas is abnormal, but the factor or factors responsible for this abnormal collagen cannot be conclusively defined at present. Collagen fibrils in leiomyomas appear widely spaced, suggesting that proteoglycans are of differing composition and/or amount compared with the adjacent myometrium. This fact alone could contribute to the abnormal appearance of the collagen fibrils observed in the leiomyoma.
Prior reports have indicated that sulfated glycosaminoglycans are altered in leiomyomas compared with myometrium. Heparin sulfate, keratin sulfate, heparin, and chondroitin sulfates were reported to be increased in leiomyomas (9). The investigators suggested that glycosaminoglycans might serve as a storage depot for growth factors and enzymes but did not mention the fact that these molecules also alter collagen fibril formation (9). The collagen-binding proteoglycan decorin colocalized with collagen type I in both leiomyomas and myometrium, and these same investigators reported that decorin was increased in leiomyomas and that the distribution of decorin was modified (10). In addition, we have found reduced expression of the collagen-binding protein dermatopontin in leiomyomas (6, 19). Either or both of these factors might account for the alteration in collagen fibrils.
The collagen cross links hydroxyproline and hydroxylysine, glycine, and proline are greater in GnRH-agonist treated leiomyomas (27), and larger fibrils (55.5 ± 5.7 vs. 45.5 ± 4) have been noted in GnRH-agonist–treated leiomyomas compared with untreated leiomyomas, along with decreased cell size and density (11). Taken together, these studies suggest that GnRH-agonist treatment may change the phenotypic expression of the smooth muscle cells, which causes altered collagen processing and altered fibrillogenesis, although definitive studies have yet to be conducted. These studies provide evidence in support of our findings that abnormal fibril formation occurs in leiomyomas.
Three prior studies have reported electron microscopy of leiomyomas. One report compared electron micrographs of a leiomyosarcoma from one subject and leiomyomas from three subjects at the time of laparotomy (12). Because the investigators focused on the smooth muscle cells of leiomyomas and leiomyosarcomas, the lower magnification (×6,500) electron micrographs did not picture the extracellular matrix in detail, making it difficult to assess the appearance of collagen fibrils. Furthermore, the investigators did not comment on the collagen fibrils other than to say that they appeared abundant. Two other studies evaluated the ultrastructure of the smooth muscle cells of leiomyomas; one study observed cultured smooth muscle cells derived from the tumors (14), and the other reported findings in tumors less than 3 mm in diameter (13). The extracellular component of fibroids was not described in either of these previous reports.
We were not surprised that our observations indicated that normal menstrual cycling did not appear to affect the collagen fibril characteristics described. Once mature collagen is formed, estrogen and P would have no effect on the fibrils. Interestingly, one convincing study has reported that leiomyomas in postmenopausal women have smaller cells than leiomyomas in premenopausal women (28). Thus, cell size could be a reason why leiomyomas shrink in menopause. That they do not disappear entirely might be caused by the fact that the fibrosis due to abnormal collagen fibrils deposition is not altered.
In conclusion, this study points to the importance of abnormally formed cross-linked collagen in the development and growth of leiomyomas. Therapeutic strategies must deal with treatment of the abnormalities of collagen formation in these very common benign tumors. Treatment strategies that will impact on the formation of the abnormal collagen fibrillogenesis in these tumors represent an exciting step forward in our search for a nonsurgical, uterus-sparing, and fertility-enhancing mode of treatment.
Acknowledgments
The authors thank Dr. Mark Payson, Matt Stenmark, Dr. Clariss Potlog-Nahari, and Melinda Sharkey for critical assistance in the collection of specimens. The authors also recognize the helpful discussions and general advice of Dr. Lynnette Nieman and Dr. John Tsibris. The continued support of Drs. George Chrousos and William Haffner assisted in completion of this work.
Supported by National Institutes of Health intramural grant no. Z01-008737-02, and in part by National Naval Medical Center grant no. B02-027.
References
- 1.Tsbris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O’Brien WF, et al. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2003;78:114–21. doi: 10.1016/s0015-0282(02)03191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Mahadevappa M, Yamamoto K, Wen Y, Chen B, Warrington JA, et al. Distinctive proliferative phase differences in gene expression in human myometrium and leiomyomata. Fertil Steril. 2003;80:266–76. doi: 10.1016/s0015-0282(03)00730-1. [DOI] [PubMed] [Google Scholar]
- 3.Catherino WH, Prupas C, Tsibris JC, Leppert PC, Payson M, Neiman LK, et al. Strategy for elucidating differentially expressed genes in leiomyomata identified by microarray technology. Fertil Steril. 2003;80:282–90. doi: 10.1016/s0015-0282(03)00953-1. [DOI] [PubMed] [Google Scholar]
- 4.Chegini N, Verala J, Luo X, Xu J, Williams RS. Gene expression profile of leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. J Soc Gynecol Investig. 2003;10:161–71. doi: 10.1016/s1071-5576(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 5.Skubitz K, Skubitz A. Differential gene expression in uterine leiomyomata. J Lab Clin Med. 2003;141:297–308. doi: 10.1016/S0022-2143(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 6.Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, et al. Reduced dermatopontin expression is a molecular link between uterine leiomyomata and keloids. Genes Chromos Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases and potentials for therapy. Ann Rev Biochem. 1995;64:403–34. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 8.Steward EA, Freidman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and type III messenger ribonucleic acids by uterine leiomyoma during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–6. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 9.Wolanska M, Sobolewski K, Drozdzewicz M, Bankowski E. Extracellular matrix components in uterine leiomyoma and their alteration during tumor growth. Mol Cell Biochem. 1998;189:145–52. doi: 10.1023/a:1006914301565. [DOI] [PubMed] [Google Scholar]
- 10.Berto AG, Sampaio LO, Franco CR, Cesar RM, Jr, Michelacci YM. A comparable analysis of structure and spatial distribution of decorin in human leiomyoma and normal myometrium. Biochimica Biophys Acta. 2003;1619:98–112. doi: 10.1016/s0304-4165(02)00446-4. [DOI] [PubMed] [Google Scholar]
- 11.Kalir T, Goldstein M, Dottino P, Brodman M, Gordon R, Deligdisch L, et al. Morphometric and electron-microscopic analysis of the effects of gonadotropin-releasing hormone agonists on uterine leiomyomas. Arch Pathol Lab Med. 1998;122:442–6. [PubMed] [Google Scholar]
- 12.Ferenczy A, Richart RM, Okagaki T. A comparative ultrastructural study of leiomyomasarcoma, cellular leiomyoma, and leiomyoma of the uterus. Cancer. 1971;28:1004–18. doi: 10.1002/1097-0142(1971)28:4<1004::aid-cncr2820280426>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Konishi I, Fujii S, Ban C, Okuda Y, Okamura H, Tojo S. Ultrastructural study of minute uterine leiomyomas. Int J Gynecol Path. 1983;2:113–20. doi: 10.1097/00004347-198302000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi K, Fujii S, Konishi I, Okamura H, Mori T. Ultrastructural study of cultured smooth muscle cells from uterine leiomyoma and myometrium under the influence of sex steroids. Gynecol Oncol. 1985;21:34–41. doi: 10.1016/0090-8258(85)90229-x. [DOI] [PubMed] [Google Scholar]
- 15.Canty EG, Kadler KE. Collagen fibril biosynthesis in tendon: a review and insights. Comp Biochem Physiol Mol Integr Physiol. 2002;133:979–85. doi: 10.1016/s1095-6433(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med. 2002;227:301–14. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- 17.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. 4 th ed. Garland Science; New York: 2002. The extracellular matrix of animals; pp. 1090–117. [Google Scholar]
- 18.Byers PH. Collagens: building blocks at the end of the development line. Clin Genet. 2000;58:270–9. doi: 10.1034/j.1399-0004.2000.580404.x. [DOI] [PubMed] [Google Scholar]
- 19.Catherino WH, Salama A, Potlog-Nahari C, Leppert PC, Tsibris JCM, Segars JH. Gene expression studies in leiomyomata: new directions for research. Sem Reprod Endocrinol. 2004;22:83–90. doi: 10.1055/s-2004-828614. [DOI] [PubMed] [Google Scholar]
- 20.Iozzo RV. The family of small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–74. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 21.Kokenysi R, Wossener JF., Jr. Effects of hormonal perturbations on the small dermatan sulfate proteoglycan and mechanical properties of the uterine cervix of late pregnant rats. Connect Tissue Res. 1991;26:199–205. doi: 10.3109/03008209109152438. [DOI] [PubMed] [Google Scholar]
- 22.Leppert PC, Kokenyesi R, Klemenich CA, Fisher J. Further evidence for a decorin-collagen interaction in the disruption of cervical collagen fibers during rat gestation. Am J Obstet Gynecol. 2000;182:805–12. doi: 10.1016/s0002-9378(00)70329-2. [DOI] [PubMed] [Google Scholar]
- 23.Keene DR, Sakai LY, Bachinger HP, Bergeson RE. Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. J Cell Biol. 1987;105:2393–402. doi: 10.1083/jcb.105.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–8. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaughan L, Mendler M, Huber S, Bruckner P, Winterhalter M, Irwin I, et al. D-periodic distribution of collagen type IX along cartilage fibrils. J Cell Biol. 1988;106:991–7. doi: 10.1083/jcb.106.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linsenmayer TF, Gibner E, Igoe F, Gordon MK, Fitch JM, Fessler LI, et al. Type V collagen: molecular structure and fibrillar organization of the chicken α1 V NH2—terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol. 1993;121:1181–9. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rein MS, Barbieri RL, Welch W, Gleason RE, Caulfield JP, Friedman AJ. The concentrations of collagen-associated amino acids are higher in GnRH agonist–treated uterine myomas. Obstet Gynecol. 1993;82:901–5. [PubMed] [Google Scholar]
- 28.Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448–53. doi: 10.5858/2000-124-1448-ROMCSA. [DOI] [PubMed] [Google Scholar]