Figure 4.
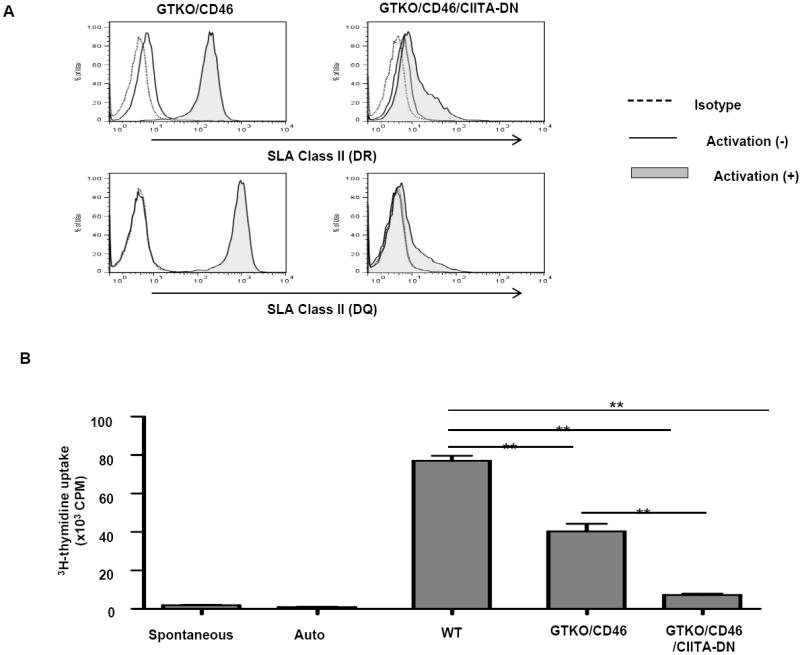
(A) Significant down-regulation of SLA class II expression on aortic endothelial cells from GTKO/CD46/CIITA-DN pigs
The expression of SLA class II DR and DQ on GTKO/CD46/CIITA-DN porcine aortic endothelial cells (pAECs) was compared with those on GTKO/CD46 pAECs. pAECs were activated with pIFN-γ (50ng/mL) for 48h. The pAECs were stained with specific anti-SLA DR or DQ mAbs. Isotype control (dotted line), quiescent (solid line), and activated pAECs (gray filled).
Expression of SLA class II on quiescent pAECs was undetectable or minimal on both GTKO/CD46 and GTKO/CD46/CIITA-DN pAECs. However, expression of SLA class II on GTKO/CD46 pAECs was significantly up-regulated when the pAECs were activated with pIFN-γ for 48h. In contrast, up-regulation of expression of SLA class II on GTKO/CD46/CIITA-DN pAECs was minimal.
(B) Significant reduction of the hCD4+T cell response to CIITA-DN cells
hCD4+T cells were co-cultured with PBMCs from WT, GTKO/CD46, and GTKO/CD46/CIITA-DN pigs for 6 days. The responses of hCD4+T cells were measured by 3H-thymidine incorporation. As a negative control, hCD4+T cells were cultured with medium only (spontaneous) or autologus PBMCs (auto). There was a significantly lower hCD4+T cell response to GTKO/CD46/CIITA-DN pig PBMCs than to either WT or GTKO/CD46 pig PBMCs (**p<0.01).
