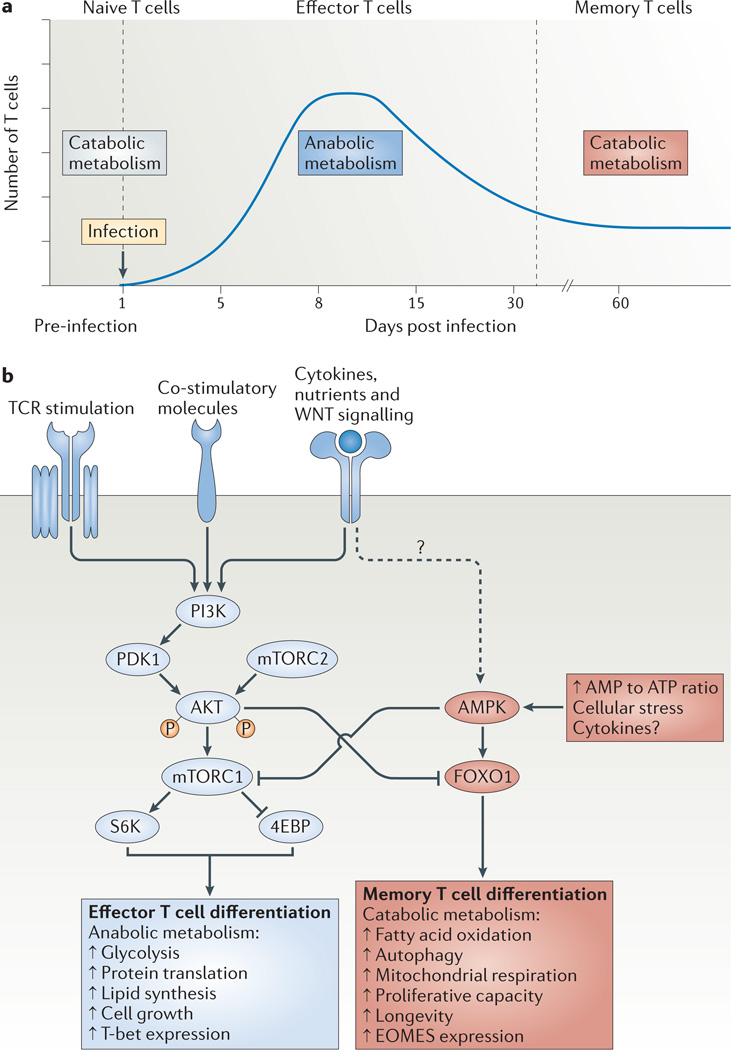
Figure 4. Model for the metabolic regulation of effector and memory T cell differentiation.
a | Resting naive or memory T cells primarily generate ATP through fatty acid oxidation and mitochondrial respiration (which are catabolic processes), but following activation the T cells undergo a metabolic switch to lipid synthesis (an anabolic process) and aerobic glycolysis to meet the bioenergetic and biosynthetic demands for rapid clonal expansion and the production of effector molecules. Following pathogen clearance, it has been proposed that effector CD8+ T cells reduce their dependence on glycolysis and are gradually ‘reset’ back to a more catabolic state to survive and further develop into memory CD8+ T cells. b | In response to T cell receptor (TCR), co-stimulatory and cytokine signals, the activity of the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathway has a key role in regulating effector CD8+ T cell metabolism and differentiation by orchestrating nutrient uptake, protein translation and lipid synthesis in rapidly proliferating effector T cells. Typically AKT functions upstream of mTOR complex 1 (mTORC1) and downstream of mTORC2, but a recent study110 has shown that S6 kinase (S6K) can be activated independently of AKT in CD8+ T cells. By contrast, cellular stress and ATP deprivation (that is, an increased AMP to ATP ratio) activate AMP-activated protein kinase (AMPK), which in turn inhibits mTOR and enhances fatty acid oxidation to cope with limiting resources. In addition, these pathways can modulate effector T cell fate decisions through transcriptional regulation. Sustained PI3K–AKT– mTOR activity inhibits forkhead box O1 (FOXO1), which acts as a molecular switch to simultaneously induce T-bet and repress eomesodermin (EOMES) expression, and thereby promotes the clonal expansion and terminal differentiation of effector T cells. 4EBP, eIF4E–binding protein; PDK1, 3-phosphoinositide-dependent protein kinase 1.