Abstract
Purpose.
Fixation stability is known to be poor for people with macular disease and has been suggested as a contributing factor for the poor visual performance of these individuals. In this study, we examined the characteristics of the different components of fixational eye movements and determined the component that plays a major role in limiting fixation stability in people with macular disease.
Methods.
Sixteen observers with macular disease and 14 older adults with normal vision (control observers) monocularly fixated a small cross presented using a Rodenstock scanning laser ophthalmoscope, for trials of 30 seconds. The retinal image and the position of the cross on the retina were recorded digitally. Eye movements were extracted from the recorded videos at a sampling rate of 540 Hz using a cross-correlation technique. A velocity criterion of 8°/s was used to differentiate between slow drifts and microsaccades.
Results.
Observers with macular disease demonstrated higher fixation instability, larger amplitudes of slow drifts and microsaccades, and lower drift velocities, when compared with older adults with normal vision. The velocity and the rate of microsaccades were comparable between the two groups of observers. Multiple linear regression analysis showed that the amplitude of microsaccades, and to a smaller extent, the amplitude of slow drifts, play a major role in limiting fixation stability.
Conclusions.
Fixation stability in people with macular disease is primarily limited by the amplitude of microsaccades, implying that rehabilitative strategies targeted at reducing the amplitude of microsaccades should improve fixation stability, and may lead to improved visual functions.
Keywords: fixational eye movements, fixation stability, low vision, macular disease
Amplitude of microsaccades plays a major role in limiting fixation stability in people with macular disease.
Introduction
Our eyes are constantly in motion even when we attempt to maintain stable fixation on a visual target. These involuntary eye movements during fixation comprise three types: tremors, slow drifts, and microsaccades. Tremors are high-frequency oscillatory motions of the eye, sometimes referred to as physiological nystagmus. Their amplitudes are small and are difficult to record accurately. Slow drifts are slow motions of the eye. For people with normal vision and oculomotor control, the amplitude of slow drifts is usually less than 10 arc min.1–8 Slow drifts are reported to account for approximately 95% of the fixation time.9 Microsaccades (sometimes known as flicks) are the fast eye movements that are interspersed among slow drifts. Although the prefix “micro” implies that the magnitude is small, in cases of patients with macular disease, the magnitude may not be small. For consistency with the literature, however, in this article we will still use the term microsaccades to refer to these fast eye movements during fixation in people with macular disease. Microsaccades are miniature saccades that account for only 3% to 5% of the fixation time.9 For people with normal vision and oculomotor control, the amplitude of microsaccades is usually less than 30 arc min and the frequency of microsaccades ranges between 0.5 and 3.0 per second.1–3,6–8,10 It is often assumed that the high-frequency tremors do not limit or contribute to the maintenance of vision, but that slow drifts and microsaccades may pose a limitation on vision.11–15
People with macular disease are known to have unstable fixation. For instance, when asked to look at a specific part of a visual target for more than several seconds, the area over which their eyes remain momentarily stationary, commonly quantified by the bivariate contour ellipse area (BCEA), can be as large as 10 to 20 deg2,16–21 more than an order of magnitude larger than that for people with intact fovea and normal vision (0.022–0.36 deg2).7,18,22,23 The high fixation instability, and the implied poor oculomotor control in people with macular disease have been suggested as the contributing factors for their poor visual acuity,24 reading ability,19,25–27 and face-recognition ability.28 As a result, there are keen interests in developing training paradigms to improve fixation stability, with the hope of improving visual functions for people with macular disease.28,29 In addition, fixation stability is now commonly used as an outcome measure to evaluate the efficacy of treatments of macular degeneration in clinical trials.30–32 Therefore, it is important to understand the fixation characteristics of people with macular disease.
Previous efforts in studying the fixation characteristics in people with macular disease have focused primarily on the location of the preferred retinal locus (PRL) for fixation and fixation stability. None have attempted to determine which parameter(s) of the fixational eye movements accounts for the high fixation instability exhibited by people with macular disease. This information is important, as it may help develop more effective eye movement training paradigms targeting at minimizing the specific component of fixational eye movements that leads to fixation instability. Therefore, the goal of this article was to examine the characteristics of fixational eye movements in people with macular disease, and to identify the component of fixational eye movements that plays a major role in limiting fixation stability in these individuals. Considering that the leading cause of macular disease is AMD, which is more prevalent in the older population, to ensure that any effect we observed was not due to age alone, we obtained measurements from a group of older adults with normal vision (control observers) for comparison.
Methods
Fixational eye movements were elicited by presenting a 1° cross (except for observers with acuity worse than 0.7 logMAR for whom we used a 2° cross) using a Rodenstock 101 scanning laser ophthalmoscope (SLO; Rodenstock, Munich, Germany) and asking observers to maintain steady fixation at the center of the cross, for trials of 30 seconds each. The SLO allows us to image the retina (field of view = 30° × 24°) in real time and the full-frame images were captured at 30 Hz. The fixation cross was presented in the primary gaze of the observers. The instruction to the observers was to “look at the center of the cross, make sure you can see the cross all the time and keep your eye as still as possible, you can blink if you need to.” Testing was monocular with the nontested eye remaining open throughout testing. Retinal images were digitally recorded for the duration of each trial by interfacing the video output of the SLO with a frame grabber (Matrox Imaging Adapter: Meteor-II PCI Frame Grabber; Matrox Electronic Systems Ltd., Dorval, QC, Canada), via a TV-One CORIO scan converter (CS-450 Eclipse, Erlanger, KY, USA). The experiment was executed and controlled using a ViSaGe system (Cambridge Research Systems, Rochester, Kent, UK), with custom-written software written in MATLAB 7.3.0 (The MathWorks, Natick, MA, USA).
Observers
Thirty observers, belonging to two different groups, participated in this study. The “macular disease” group consisted of 16 observers (eight males and eight females, mean age = 75.1, range 48–89), and the “older adults” group consisted of 14 older adults with normal vision (seven males and seven females, mean age = 69.3, range 62–77). The visual characteristics of the 16 observers in the macular disease group are given in Table 1. The PRL location was provided only for the tested eye (usually the better-seeing eye). The duration of vision loss (years since onset) is given in Table 1. In general, there was no correlation between the years since onset with visual acuity or the PRL location for these observers. For observers in the older group, one eye was randomly chosen as the tested eye.
Table 1.
Visual Characteristics of the 16 Observers With Macular Disease
|
Observer |
M/F |
Age, y |
Diagnosis |
Years Since Onset |
Eye |
Acuity, logMAR |
PRL ecc, deg |
| 1 | F | 86 | AMD | 11 | OD | 1.12 | 12.07 |
| OS | Hand motion | ||||||
| 2 | M | 56 | Stargardt | 38 | OD | 1.02 | 8.41 |
| OS | 1.04 | ||||||
| 3 | M | 57 | Stargardt | 40 | OD | 1.1 | |
| OS | 1.1 | 12.73 | |||||
| 4 | F | 82 | AMD | 9 | OD | 0.5 | 2.70 |
| OS | 0.52 | ||||||
| 5 | F | 74 | AMD | 6 | OD | 0.54 | 1.39 |
| OS | 1.12 | ||||||
| 6 | M | 85 | AMD | 11 | OD | 0.7 | 4.08 |
| OS | 0.74 | ||||||
| 7 | M | 78 | AMD | 9 | OD | 0.62 | 3.57 |
| OS | 0.86 | ||||||
| 8 | F | 73 | AMD | 7 | OD | 0.66 | |
| OS | 0.48 | 1.33 | |||||
| 9 | F | 78 | AMD | 3 | OD | 0.4 | 2.01 |
| OS | 0.32 | ||||||
| 10 | M | 48 | Stargardt | 26 | OD | 1 | |
| OS | 0.98 | 5.36 | |||||
| 11 | F | 79 | AMD | 8 | OD | 0.74 | 1.66 |
| OS | 0.92 | ||||||
| 12 | F | 77 | AMD | 5 | OD | 1.12 | |
| OS | 1.08 | 10.33 | |||||
| 13 | M | 72 | AMD | 4 | OD | 1.1 | |
| OS | 1.02 | 7.81 | |||||
| 14 | F | 89 | AMD | 15 | OD | 1 | 3.39 |
| OS | 1.02 | ||||||
| 15 | M | 84 | AMD | 8 | OD | 0.56 | 4.68 |
| OS | 0.7 | ||||||
| 16 | M | 84 | AMD | 19 | OD | 0.44 | |
| OS | 0.5 | 1.77 |
Observers in the older adults group had a complete eye examination within the 2 years before the experiment. All had best-corrected distance visual acuity of 20/20 (logMAR acuity 0.0) or better in each eye, no signs or symptoms of any eye disease or disorder, and normal oculomotor control. Observers in the macular disease group were referred from the Low Vision Clinic at the University of Houston College of Optometry or the University of California Berkeley School of Optometry. All had a complete low-vision evaluation within the 3 months before their participation in the experiment, and all had macular lesions due to AMD or Stargardt disease, but none of them was under any form of active treatment for their condition. All observers, regardless of the group to which they belonged, gave informed oral and written consent after the procedures of the experiment were explained and before the commencement of data collection. The research followed the tenets of the Declaration of Helsinki and was approved by the Committee for Protection of Human Subjects at the University of Houston and the University of California, Berkeley.
Extraction of Eye Position From Video Files
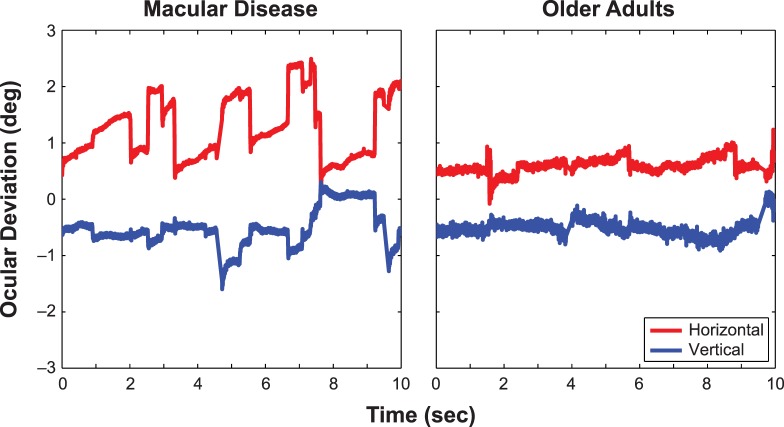
For each trial, fixational eye movements were extracted from the raw video of retinal images using a cross-correlation procedure as described by Stevenson and Roorda.33 This procedure takes advantage of the facts that each scan line of a video frame was collected at a different time from the other scan lines of the same video frame and the eye undergoes an extremely small amount of motion within a scan line (especially when compared with the motion in an entire frame). Therefore, by determining the relative shifts in position of different image features within the same video frame, we could recover eye position at a sampling rate much higher than the 30 Hz at which the videos were recorded. For each video, we first constructed a reference image based on a subset of frames (∼20–40) with good image quality extracted from the 900 recorded frames (30 seconds of recording at 30 Hz). This subset of good frames was chosen based on their image quality, and excluded frames in which images were lost due to eye blinks or poor image quality. A cross-correlation procedure was used to combine these good frames into the reference image for subsequent data analysis. Then we extracted an epoch of the first 10 seconds of the recorded video for the analysis of the various parameters of the fixational eye movements. To do so, for each video frame to be analyzed, we divided each frame into horizontal strips, each consisting of 15 scan lines, and used 14 of them (evenly spaced vertically) for the cross-correlation analysis (there were in fact a total of 18 strips for each video frame but strip numbers 15 through 18 were contained in the scanner fly back and did not contain any useful retinal information). We cross-correlated each strip with the reference image to determine its horizontal and vertical position at the time that the scan lines were obtained. By analyzing individual strips of the same video frame, we effectively sampled the eye movement data at 540 Hz (note that the sampling rate was 960 Hz in Stevenson and Roorda33 using their parameters). Figure 1 shows the horizontal and vertical eye position traces for a 10-second epoch from an observer in each of the two groups (the one with fixation stability quantified by BCEA closest to the median value in the respective group).
Figure 1.
Eye position traces of two observers, whose fixation stability (according to BCEA) was the median of their respective groups, are shown here. For clarity, the horizontal (red) and vertical (blue) eye position traces are offset vertically in each panel.
Quantifying Fixation Stability
After the horizontal and vertical eye positions were recovered from each video, we calculated the fixation stability by using two different methods. The first one was the conventional method of quantifying fixation stability using BCEA, based on the eye positions over a 10-second epoch, where BCEA (in deg2) is defined as
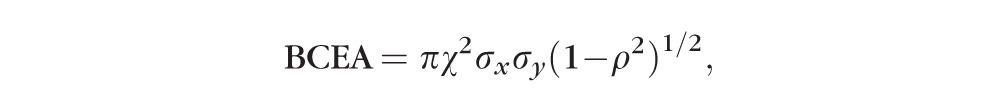 |
where χ2 is the Chi-squared value corresponding to a probability of 0.68, σx and σy are the SDs in the horizontal (x) and vertical (y) directions, and ρ is the Pearson product moment correlation coefficient between x and y. However, as the BCEA assumes a normal distribution of eye positions, an assumption that is usually violated in the case of fixations in people with macular disease, and that the BCEA includes (and does not differentiate between) the variability between and within fixations,34 we also calculated fixation variability based on the isoline method that does not require a normal distribution of eye positions or that observers use a single location for fixation.34,35 To do so, we first estimated the probability density function corresponding to the eye position samples by using kernel density estimation. Then we chose a level of density corresponding to 68% of the data points. The area enclosed by this 68% isoline was then calculated.
Slow Drifts and Fixation Saccades
Given that the resolution of the SLO does not allow us to accurately record tremors, we examined only slow drifts and microsaccades. Microsaccades were identified when three consecutive samples of eye positions were in the same direction of motion, and that the velocity between samples was 8 deg/s or more and either had the same or an increasing magnitude as the sample before. The first sample in this case was taken as the starting point of a microsaccade. We defined the end point of this microsaccade as the first sample of eye position when the velocity between this and the subsequent sample fell below 8 deg/s. Traditionally, a fixed velocity criterion has been used to differentiate between microsaccades and slow drifts.36,37 Here, we updated the value of the velocity criterion to reflect the range of reported velocities of microsaccades when bite bars were not used to stabilize the head motion.14 Before saccade detection, the eye position traces were smoothed with a five-sample moving average filter to reduce the effects of noise. The filtered traces were used only to identify saccades, all subsequent analyses were performed on unfiltered traces. Slow drifts were defined as the eye movements between two successive microsaccades, but our analysis was performed only on the position samples that excluded the first five position samples following the end of one microsaccade and the last five position samples before the beginning of the next microsaccade. In this article, we focus our comparisons on the following parameters: rate of microsaccades, velocities of the two-dimensional vector of slow drifts and microsaccades (averaged velocity across samples), and amplitudes of slow drifts and microsaccades. The rate of microsaccades could limit fixation stability if the direction of microsaccades is not random, but instead there is a predominant direction of motion. In this case, the spatial region over which the eye lands would increase, thus increasing the fixation instability. The velocity of slow drifts and microsaccades could limit fixation stability because for a fixed amplitude and rate of occurrences, the landing position of the eye would be farther from the starting position for a higher-velocity slow drift or microsaccade than for a slower-velocity one, thus increasing the fixation instability. Similarly, if other parameters remain the same, then a larger-amplitude slow drift or microsaccade would also land the eye farther away from the starting position than one with a smaller amplitude; again, increasing the fixation instability. All the values reported represent the averaged values of the measurements across multiple occurrences (e.g., multiple microsaccades in each trial) and multiple trials, for each eye/observer. Although the findings reported here were obtained using a velocity criterion of 8 deg/s to classify eye movements into slow drifts and microsaccades, when we repeated our analyses using a velocity criterion of 15 deg/s, the results were qualitatively similar (the values of all parameters changed, of course) and did not alter our main findings and conclusions.
Results
We used box plots to present our main comparisons between the two groups of observers, and the Mann-Whitney U test to determine if the two distributions were similar. All the probabilities reported were for a two-tailed comparison, with the two sample sizes being 16 (n1) and 14 (n2), for the macular disease and the older adult group, respectively. All statistical analyses reported in this article were performed using the R free software (http://www.R-project.org [in the public domain]; R Development Core Team, Vienna, Austria).38
People with macular diseases are known to have poor fixation stability. Using our cross-correlation procedure to recover eye positions, we found that the median value of BCEA (calculated for χ2 = 0.68) was approximately 25 times higher for the macular disease group than for the older adults (1.99 vs. 0.08 deg2, Fig. 2, left). The range of BCEA values for observers with macular disease was 0.09 to 8.71 deg2, consistent with the values reported for individuals with macular disease (from near-normal values to 20 deg2),16–21 whereas the range for the older adults was 0.025 to 0.17 deg2, also consistent with the values reported for individuals with normal vision (0.022–0.36 deg2).7,18,22,23,39 The Mann-Whitney U test, performed on the log value of the BCEA, showed that the distributions of the two groups differed significantly (U = 220, P < 0.0001).
Figure 2.
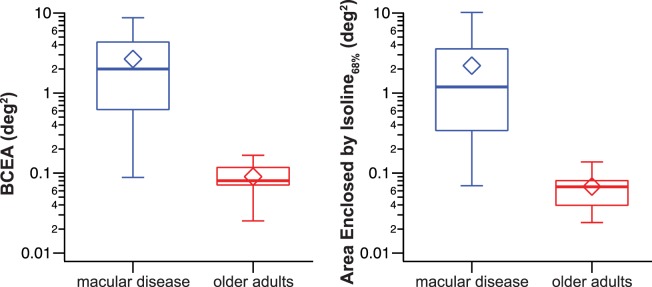
Box-and-whisker plots comparing fixation stability, quantified as BCEA (left) or the area enclosed by the isoline corresponding to 68% of the probability density function of the eye position distribution (right), for the two groups of observers. The upper and lower bound of each box represent the 75th and 25th percentiles of the distribution, and the median is represented by the thick line inside the box. The top and bottom ends of the whisker represent the 95th and 5th percentiles of the distribution, respectively. The diamond symbol represents the mean value.
When fixation stability was calculated based on the isoline that corresponded to the 68% of the probability density function estimated for the eye position data, we found that the result was qualitatively similar. The median value was approximately 17 times higher for the macular disease group than for the older adult group (1.19 vs. 0.07 deg2, Fig. 2, right). The Mann-Whitney U test, performed on the log value of these data, confirmed that the distributions of the two groups differed significantly (U = 220, P < 0.0001).
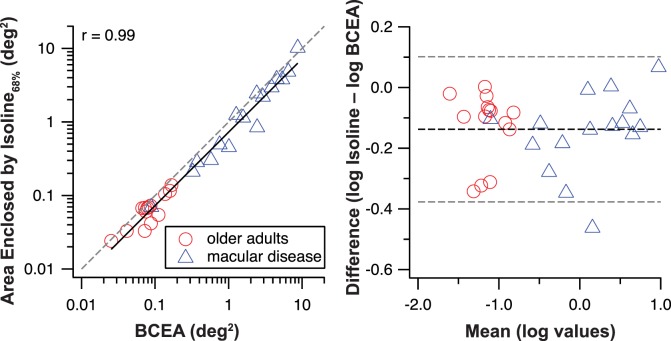
The similarity between the left and right panels of Figure 2 is interesting, because previous studies have pointed out that the use of BCEA to quantify fixation stability assumes that the eye positions during fixation follow a normal distribution,39 whereas the isoline method does not make this assumption.34,35 Therefore, one would expect that the results from the two methods do not necessarily correlate or agree with one another. Contrary to the expectation, the values obtained using the two methods are highly correlated (r = 0.99) with one another, as shown in the left panel of Figure 3. The slope (on log–log axes) of the regression line is very close to 1 (0.99) but there is a constant offset from the 1:1 line (gray dashed line), implying that the isoline values are smaller than the BCEA by a similar amount across observers (normally sighted or with macular disease). Considering that a high correlation does not imply a high agreement, we also examined how well the values obtained using the two methods agree with one another, as shown in a Bland-Altman plot40 (Fig. 3, right). Given that all except one data point fall within ±95% limits of agreement, we conclude that the BCEA and the isoline methods agree reasonably well in representing fixation stability, with the caveat that the isoline yields slightly better stability (the mean difference between the log values of the two methods = 0.138 log deg2, which is statistically different from a null effect [no difference], P < 0.0001). The result that the values obtained using the two methods are highly correlated and in excellent agreement with one another is unexpected, and there are two noteworthy points in relation to these findings. First, none of our observers, with or without macular disease, showed evidence of using more than one retinal location as their fixation locus, as revealed by the recorded SLO video files. Second, none of our observers had eye positions during fixation that followed a normal distribution, based on the Kolmogorov-Smirnov test to test for normality in the data. Although this was expected for people with macular disease, our finding showed that this was also true for older adults with normal vision, at least using our method of eye movement measurements. We believe these two points are likely to account for the high correlation and agreement observed between the values obtained using the two methods, but more detailed analyses and further studies are necessary for us to fully understand why the values from the two methods are so similar. The important point in relation to the purpose of this study is that using the two different methods to quantify fixation stability, observers with macular disease consistently demonstrated much higher fixation instability than older adults with normal vision.
Figure 3.
Left: Fixation stability quantified by the area enclosed by the isoline is plotted as a function of BCEA. The data show a very high correlation and the slope of the line (on log–log axes) is 0.99. However, there is a constant offset from the 1:1 line (gray dashed line). Right: The isoline and BCEA values are compared in a Bland-Altman plot. The mean difference (using log values) between the two methods is statistically different from 0 (represented by the middle black dashed line), consistent with the left panel that the isoline values are smaller than the BCEA values. All except one data point falls within the ±95% limits of agreement (gray dashed lines).
The much higher fixation instability demonstrated by observers with macular disease is not surprising, but which component(s) of the fixational eye movements contributes to the fixation instability? To answer this question, we first compared the rate of microsaccades, the vector velocity, and the amplitude of slow drifts and microsaccades between the two groups to determine which characteristics are different. Then we examined if the characteristics that are different between the two groups show a significant correlation with fixation stability.
Figure 4 compares the rate of microsaccades (median: 2.48 vs. 1.49 per second) between the macular disease and the older adult groups. The distributions of the two groups were not different (U = 154.5, P = 0.080), implying that the increased fixation instability in observers with macular disease cannot be attributed to an increased rate of microsaccades.
Figure 4.
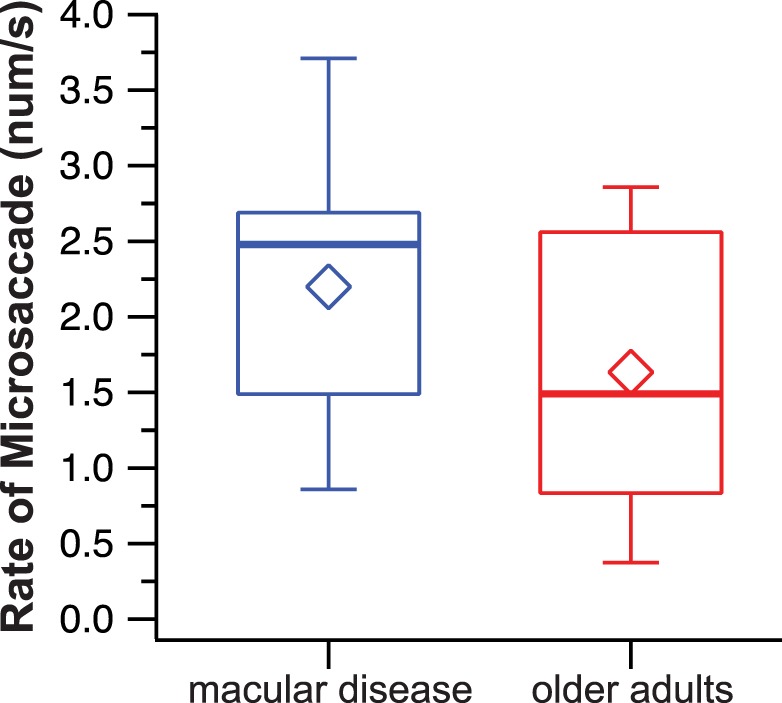
Box-and-whisker plots comparing the number of microsaccades per second between the two groups of observers. Details of the box and whiskers are as in Figure 2.
If the increased fixation instability in observers with macular disease is not due to an increased rate of microsaccades, could it be due to the faster velocity or larger amplitude of either the slow drifts and/or the microsaccades? Although the median velocities of slow drifts were quite similar at 5.13 and 5.39 deg/s for the macular disease and the older adult groups (Fig. 5, left), the distributions in the two groups differed significantly (U = 31, P = 0.0004). Note that in this case, the slow drift velocity was slower in the macular disease group. For microsaccades, the median velocities were 50.7 deg/s for the macular disease group, versus 40.6 deg/s for the older adults group (Fig. 5, right), and the distributions in the two groups did not differ significantly (U = 154, P = 0.085).
Figure 5.
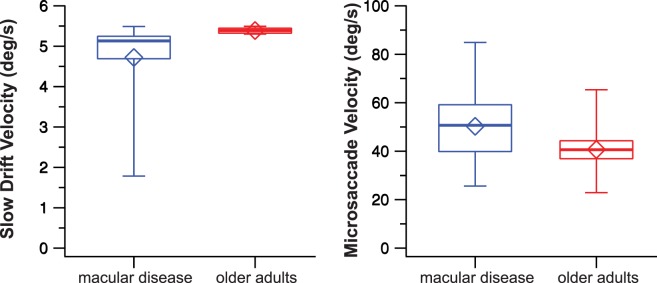
Box-and-whisker plots comparing the vector velocities of slow drifts (left) and microsaccades (right) between the two groups of observers. Details of the box and whiskers are as in Figure 2.
Besides velocities, the distributions of the amplitudes of slow drifts and microsaccades in the two groups were also significantly different (Fig. 6). For slow drifts, the median amplitude was almost twice as large in the macular disease group than in the older adult group (13.84 vs. 7.64 arc min; U = 218, P < 0.0001). Similarly, the median amplitude of microsaccades was a factor of 3.5 larger in the macular disease group than in the older adult group (52.96 vs. 14.96 arc min; U = 210, P < 0.0001). Note that for our older adults with normal vision, their amplitudes of slow drifts and microsaccades all fell within the range previously reported for people with normal vision.1–8,10
Figure 6.
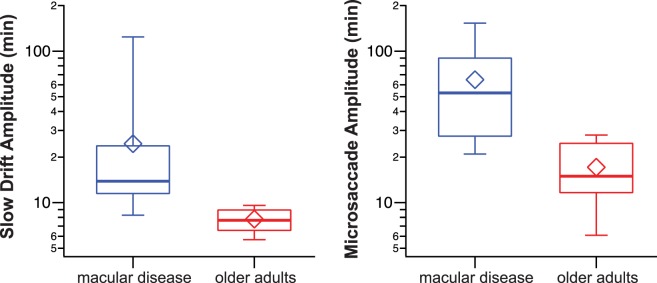
Box-and-whisker plots comparing the amplitudes of slow drifts (left) and microsaccades (right) between the two groups of observers. Details of the box and whiskers are as in Figure 2.
These results suggest that the higher fixation instability exhibited by observers with macular disease could be due to the larger amplitudes of slow drifts and/or microsaccades, and maybe the slower velocity of slow drifts made by these observers, when compared with the older adults. If so, then we would expect a correlation between fixation instability and each of these variables. Considering that BCEA is highly correlated and agreed well with the area enclosed by the isoline that corresponded to 68% of the probability density function of the eye position data (Fig. 2), here we use only BCEA to quantify fixation stability. Figure 7 plots BCEA as a function of the amplitude of microsaccades (top), amplitude of slow drifts (middle), and the velocity of slow drifts (bottom). The straight line in each panel represents the best-fit regression line (on log–log axes) to the data pooled across the two groups of observers, for each individual variable considered alone. In general, BCEA shows a high correlation with the amplitude of microsaccades (r = 0.91) and slow drifts (r = 0.70), but not with the velocity of slow drifts, suggesting that the amplitudes of microsaccades and slow drifts are likely to be the important factors limiting fixation stability.
Figure 7.
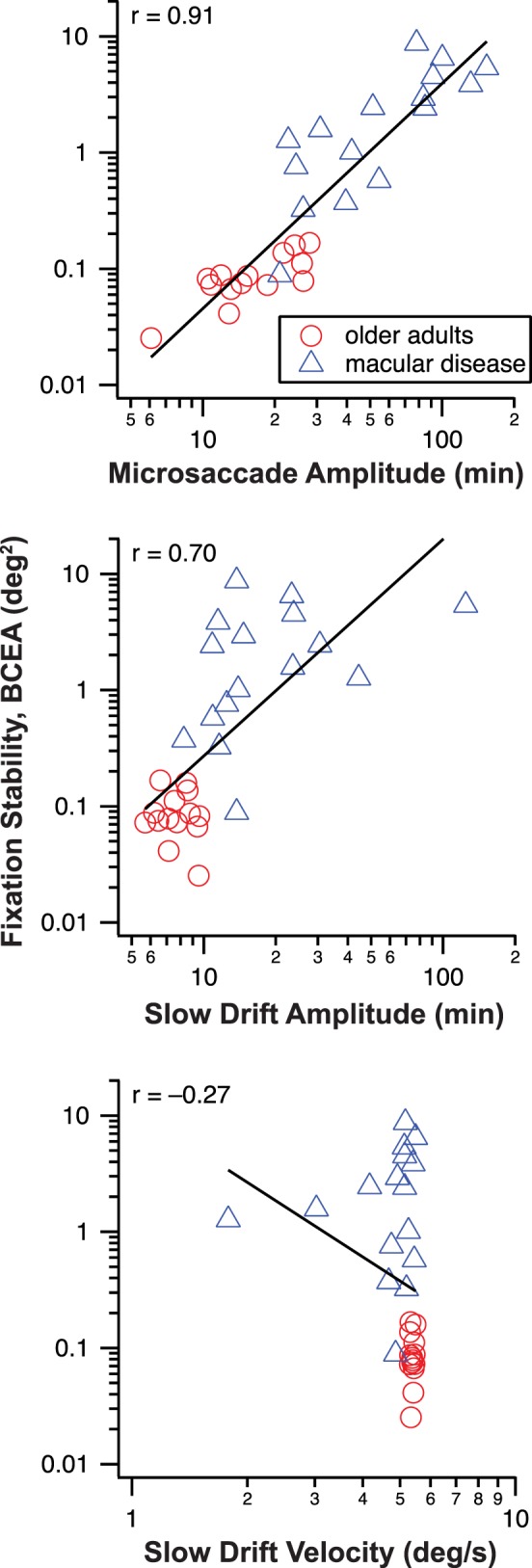
Fixation stability quantified as BCEA (deg2) is plotted as a function of the amplitude of microsaccade (top), amplitude of slow drifts (middle), and velocity of slow drifts (bottom). The straight line in each panel is the best-fit regression line to the data (log–log axes), with the correlation coefficient given in the upper left corner in each panel.
To determine how fixation stability relates to the combined effects of the amplitude of microsaccade and the amplitude of slow drifts, we performed a multiple linear regression analysis. We also added the observer grouping (older adults = 0; macular disease = 1) as a factor because it is clear that fixation stability is different between older adults and observers with macular disease. The results of the multiple regression analysis are summarized in Table 2. The effects of the amplitude of microsaccade (P < 0.0001), amplitude of slow drifts (P = 0.0454), and observer grouping (P = 0.0128) are all significant: in other words, each of these factors has a unique effect on BCEA (i.e., each effect is significant even when the effects of the other two factors are controlled for). The combination of these variables yields a multiple r2 of 0.901, and an adjusted r2 of 0.889. However, which of these factors is the most important to predict fixation stability and which is the least important? Using the relaimpo package in R and the “lmg” metric,41 the amplitude of microsaccade ranked top in terms of relative importance (accounting for 46.8% [95% confidence intervals: 36.1%–58.3%] of r2, followed by observer grouping (32.8% [22%–42%] and the amplitude of slow drifts (20.4% [15.0%–30.3%]). In sum, these results imply that the amplitude of microsaccade is the most important oculomotor parameter in limiting fixation stability, and although the amplitude of slow drifts can also limit fixation stability, its effect is much weaker. Note also that whether someone has macular disease or not also strongly influences fixation stability, as those with macular disease usually have poorer fixation stability, but this result has already been widely reported in the past.
Table 2.
Results of the Multiple Regression of Fixation Stability (BCEA) on Microsaccade Amplitude, Slow Drift Amplitude, and Observer Group (With Macular Disease or Not)
|
Estimate |
SE |
t
Value |
Pr(>|t|) |
|
| Intercept | −3.046 | 0.292 | −10.434 | <0.00001 |
| Microsaccade amplitude | 1.290 | 0.201 | 6.409 | <0.00001 |
| Slow drift amplitude | 0.465 | 0.221 | 2.102 | 0.0454 |
| Observer grouping | 0.415 | 0.155 | 2.673 | 0.0128 |
Residuals SE: 0.256 on 26 df. Multiple R2 = 0.901; adjusted R2 = 0.889 (P < 3.67e−13).
Discussion
The primary goal of this study was to examine the characteristics of fixational eye movements in people with macular disease, and to identify the component of fixational eye movements that plays a major role in limiting fixation stability in these individuals. We found that the rate of microsaccades and the velocity of microsaccades are not different between the groups of observers with macular disease and older adults with normal vision; thus, neither of these can account for the high fixation instability exhibited by observers with macular disease. The velocities of slow drifts exhibited for the observers in the macular disease group are found to be statistically slower than those in the older adults group, but they do not show a correlation with fixation stability. The two oculomotor parameters that show a significant relationship with fixation stability in a multiple regression analysis are the amplitude of microsaccades and the amplitude of slow drifts, with the amplitude of microsaccades being the more important factor. Based on our findings, we conclude that the high fixation instability in people with macular disease is largely due to the large amplitude of microsaccades, and to a smaller extent, the amplitude of slow drifts.
There are at least two implications of the finding that the high fixation instability in people with macular disease is attributed to the large amplitudes of microsaccades and slow drifts but not the other parameters of fixational eye movements. First, it is often assumed that high fixation instability is associated with increased retinal image motion.42 If retinal image motion is defined based on the retinal image velocity, then the assumption of high fixation instability being associated with increased retinal image motion, or velocity, is incorrect, because there is no correlation between the velocities of slow drifts or microsaccades and fixation stability. In a previous study, Macedo et al.42 compensated for various degrees of retinal image velocities due to fixational eye movements in a group of observers with macular disease and found that in general acuity was not affected by the retinal image velocities associated with fixational eye movements. They concluded that “fixation instability does not improve visual acuity.” However, their manipulation of the stimulus only changed the velocities, not fixation stability. Hence, their results would be better interpreted as evidence that the retinal image velocity associated with fixational eye movements does not affect visual acuity.
The second implication relates to the training of fixational eye movements as a rehabilitative paradigm for patients with macular disease.43,44 Given our findings, any training paradigm that aims at improving fixation stability should target at reducing the amplitude of microsaccades. An example of a technique that may achieve this goal is auditory biofeedback. Auditory biofeedback has been used or suggested as a treatment option for other clinical conditions, such as amblyopia45 and congenital nystagmus,46,47 to improve fixation stability through a reduction in the amplitude of the eye movement. Recently, auditory feedback has been used to improve fixation stability in people with macular disease,43,44 although it is unclear from these studies whether the increased fixation stability observed in these studies was simply due to a relocation of the PRL,43 reduced amplitude of eye movements, or an increased attention modulation.44 Further studies are required to examine the cause of the improvement in fixation stability observed in people with macular disease using auditory biofeedback.
A caveat about reducing the amplitude of microsaccades through training is that to date, we still do not have a concrete understanding of the functional role of microsaccades. At least for people with normal vision, whether or not microsaccades are beneficial, detrimental, or serve no purpose to vision is still under debate.13–15,48–50 Logically, this also implies that we do not know if microsaccades serve any purpose for the maintenance of vision for people with macular disease. Considering that all of our observers with macular disease had a well-established PRL for fixation, which were located in the peripheral retina where the cone spacing is larger and the ratio of cones to ganglion cell is greater than that at the fovea, the larger amplitude of microsaccades may help to ensure that the image of a visual target lands in different (groups of) cone photoreceptors (or sampling units) from one finite time period to another, so that the visual target will always be refreshed on a different group of photoreceptors (or sampling unit) and will not fade away. If this is indeed the case, then reducing the amplitude of microsaccades through training may not be beneficial to people with macular disease.
Acknowledgments
The authors thank Yiji Lin for technical support and Eric Castet for sharing his R script in calculating fixation stability using the isoline method.
Supported by National Institutes of Health Grant R01-EY012810.
Disclosure: G. Kumar, None; S.T.L. Chung, None
References
- 1. Ratliff F, Riggs LA. Involuntary motions of the eye during monocular fixation. J Exp Psychol. 1950; 40: 687–701 [DOI] [PubMed] [Google Scholar]
- 2. Ditchburn RW, Ginsborg BL. Involuntary eye movements during fixation. J Physiol. 1953; 119: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riggs LA, Armington JC, Ratliff F. Motions of the retinal image during fixation. J Opt Soc Am. 1954; 44: 315–321 [DOI] [PubMed] [Google Scholar]
- 4. Krauskopf J, Cornsweet TN, Riggs LA. Analysis of eye movements during monocular and binocular fixation. J Opt Soc Am. 1960; 50: 572–578 [DOI] [PubMed] [Google Scholar]
- 5. Nachmias J. Determiners of the drift of the eye during monocular fixation. J Opt Soc Am. 1961; 51: 761–766 [DOI] [PubMed] [Google Scholar]
- 6. Ditchburn RW, Foley-Fisher JA. Assembled data on eye movements. Optica Acta. 1967; 14: 113–118 [DOI] [PubMed] [Google Scholar]
- 7. Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1975; 181: 810–819 [DOI] [PubMed] [Google Scholar]
- 8. Sansbury RV, Skavenski AA, Haddad GM, Steinman RM. Normal fixation of eccentric targets. J Opt Soc Am. 1973; 63: 612–614 [DOI] [PubMed] [Google Scholar]
- 9. Yarbus AL. Eye Movements and Vision. New York: Plenum; 1967. [Google Scholar]
- 10. Cherici C, Kuang X, Poletti M, Rucci M. Precision of sustained fixation in trained and untrained observers. J Vis. 2012; 12: 31.1–31.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cornsweet TN. Determination of the stimuli for involuntary drifts and saccadic eye movements. J Opt Soc Am. 1956; 46: 987–993 [DOI] [PubMed] [Google Scholar]
- 12. Gerrits HJ, Vendrik AJ. The influence of stimulus movements on perception in parafoveal stabilized vision. Vision Res. 1974; 14: 175–180 [DOI] [PubMed] [Google Scholar]
- 13. Ditchburn RW. The function of small saccades. Vision Res. 1980; 20: 217–272 [DOI] [PubMed] [Google Scholar]
- 14. Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nat Rev Neurosci. 2004; 5: 229–240 [DOI] [PubMed] [Google Scholar]
- 15. Ko HK, Poletti M, Rucci M. Microsaccades precisely relocate gaze in a high visual acuity task. Nat Neurosci. 2010; 13: 1549–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White JM, Bedell HE. The oculomotor reference in humans with bilateral macular disease. Invest Ophthalmol Vis Sci. 1990; 31: 1149–1161 [PubMed] [Google Scholar]
- 17. Schuchard RA, Fletcher DC. Preferred retinal locus: a review with applications in low vision rehabilitation. Ophthalmol Clin North Am. 1994; 7: 243–256 [Google Scholar]
- 18. Rohrschneider K, Becker M, Kruse FE, Fendrich T, Vicker HE. Stability of fixation: results of fundus-controlled examination using the scanning laser ophthalmoscope. Ger J Ophthalmol. 1995; 4: 197–202 [PubMed] [Google Scholar]
- 19. Crossland MD, Culham LE, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic Physiol Opt. 2004; 24: 327–333 [DOI] [PubMed] [Google Scholar]
- 20. Timberlake GT, Sharma MK, Grose SA, Gobert DV, Gauch JM, Maino JH. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optom Vis Sci. 2005; 82: 177–185 [DOI] [PubMed] [Google Scholar]
- 21. Crossland MD, Crabb DP, Rubin GS. Task-specific fixation behavior in macular disease. Invest Ophthalmol Vis Sci. 2011; 52: 411–416 [DOI] [PubMed] [Google Scholar]
- 22. Kosnik W, Fikre J, Sekuler R. Visual fixation stability in older adults. Invest Ophthalmol Vis Sci. 1986; 27: 1720–1725 [PubMed] [Google Scholar]
- 23. Crossland MD, Rubin GS. The use of an infrared eyetracker to measure fixation stability. Optom Vis Sci. 2002; 79: 735–739 [DOI] [PubMed] [Google Scholar]
- 24. Reinhard J, Messias A, Dietz K, et al. Quantifying fixation in patients with Stargardt disease. Vision Res. 2007; 47: 2076–2085 [DOI] [PubMed] [Google Scholar]
- 25. Rubin GS, Feely M. The role of eye movements during reading in patients with age-related macular degeneration (AMD). Neuro-Ophthalmology. 2009; 33: 120–126 [Google Scholar]
- 26. Seiple W, Grant P, Szlyk JP. Reading rehabilitation of individuals with AMD: relative effectiveness of training approaches. Invest Ophthalmol Vis Sci. 2011; 52: 2938–2944 [DOI] [PubMed] [Google Scholar]
- 27. Amore FM, Fasciani R, Silvestri V, et al. Relationship between fixation stability measured with MP-1 and reading performance. Ophthalmic Physiol Opt. 2013; 33: 611–617 [DOI] [PubMed] [Google Scholar]
- 28. Seiple W, Rosen RB, Garcia PM. Abnormal fixation in individuals with age-related macular degeneration when viewing an image of a face. Optom Vis Sci. 2013; 90: 45–56 [DOI] [PubMed] [Google Scholar]
- 29. Seiple W, Szlyk JP, McMahon T, Pulido J, Fishman GA. Eye-movement training for reading in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005; 46: 2886–2896 [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez EG, Tarita-Nistor L, Mandelcorn ED, Mandelcorn M, Steinbach MJ. Fixation control before and after treatment for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 4208–4213 [DOI] [PubMed] [Google Scholar]
- 31. Pearce E, Sivaprasad S, Chong NV. Comparing fixation location and stability in patients with neovascular age-related macular degeneration treated with or without Ranibizumab. Eye (Lond). 2011; 25: 149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anastassiou G, Schneegans AL, Selbach M, Kremmer S. Transpalpebral electrotherapy for dry age-related macular degeneration (AMD): an exploratory trial. Restor Neurol Neurosci. 2013; 31: 571–578 [DOI] [PubMed] [Google Scholar]
- 33. Stevenson SB, Roorda A. Correcting for miniature eye movements in high resolution scanning laser ophthalmoscopy. In: Manns F, Soderberg P, Ho A. eds Ophthalmic Technologies XV: Proceedings of the SPIE. Bellingham, WA: SPIE; 2005: 145–151 [Google Scholar]
- 34. Castet E, Crossland M. Quantifying eye stability during a fixation task: a review of definitions and methods. Seeing Perceiving. 2012; 25: 449–469 [DOI] [PubMed] [Google Scholar]
- 35. Whittaker SG, Budd J, Cummings RW. Eccentric fixation with macular scotoma. Invest Ophthalmol Vis Sci. 1988; 29: 268–278 [PubMed] [Google Scholar]
- 36. St Cyr GJ, Fender DH. The interplay of drifts and flicks in binocular fixation. Vision Res. 1969; 9: 245–265 [DOI] [PubMed] [Google Scholar]
- 37. Boyce PR. Monocular fixation in human eye movement. Proc R Soc Lond B. 1967; 167: 293–315 [DOI] [PubMed] [Google Scholar]
- 38. R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. Available at: http://www.R-project.org [Google Scholar]
- 39. Steinman RM. Effect of target size, luminance, and color on monocular fixation. J Opt Soc Am. 1965; 55: 1158–1165 [Google Scholar]
- 40. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1: 307–310 [PubMed] [Google Scholar]
- 41. Grömping U. Relative importance for linear regression in R: the package relaimpo. J Stat Soft. 2006; 17: 1–27 [Google Scholar]
- 42. Macedo AF, Crossland MD, Rubin GS. Investigating unstable fixation in patients with macular disease. Invest Ophthalmol Vis Sci. 2011; 52: 1275–1280 [DOI] [PubMed] [Google Scholar]
- 43. Tarita-Nistor L, Gonzalez EG, Markowitz SN, Steinbach MJ. Plasticity of fixation in patients with central vision loss. Vis Neurosci. 2009; 26: 487–494 [DOI] [PubMed] [Google Scholar]
- 44. Vingolo EM, Salvatore S, Cavarretta S. Low-vision rehabilitation by means of MP-1 biofeedback examination in patients with different macular diseases: a pilot study. Appl Psychophysiol Biofeedback. 2009; 34: 127–133 [DOI] [PubMed] [Google Scholar]
- 45. Flom MC, Kirschen DG, Bedell HE. Control of unsteady, eccentric fixation in amblyopic eyes by auditory feedback of eye position. Invest Ophthalmol Vis Sci. 1980; 19: 1371–1381 [PubMed] [Google Scholar]
- 46. Ciuffreda KJ, Goldrich SG, Neary C. Use of eye movement auditory biofeedback in the control of nystagmus. Am J Optom Physiol Opt. 1982; 59: 396–409 [DOI] [PubMed] [Google Scholar]
- 47. Kirschen DG. Auditory feedback in the control of congenital nystagmus. Am J Optom Physiol Opt. 1983; 60: 364–368 [DOI] [PubMed] [Google Scholar]
- 48. Rolfs M. Microsaccades: small steps on a long way. Vision Res. 2009; 49: 2415–2441 [DOI] [PubMed] [Google Scholar]
- 49. Kowler E, Steinman RM. Miniature saccades: eye movements that do not count. Vision Res. 1979; 19: 105–108 [DOI] [PubMed] [Google Scholar]
- 50. Kowler E, Steinman RM. Small saccades serve no useful purpose: reply to a letter by R. W. Ditchburn. Vision Res. 1980; 20: 273–276 [DOI] [PubMed] [Google Scholar]