Fig. 6.
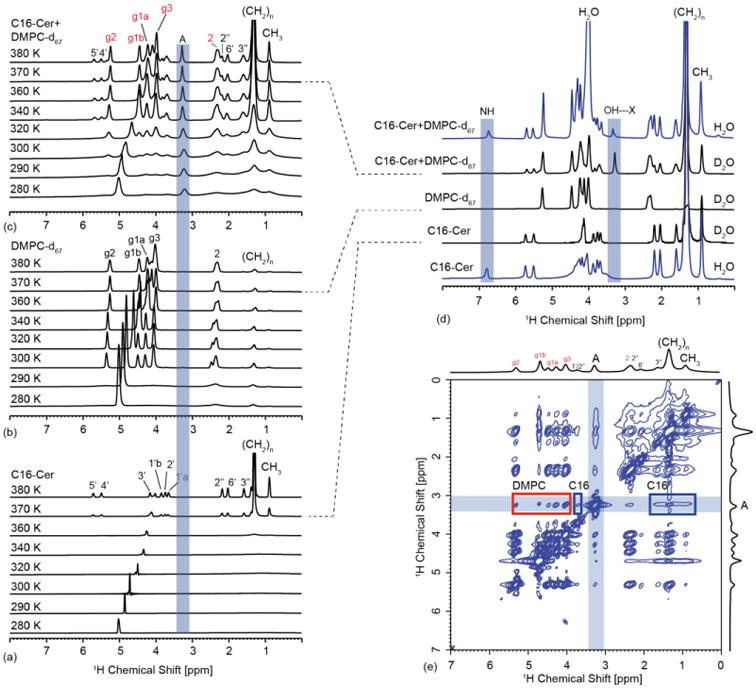
(a) Temperature dependent 1H-MAS NMR spectra of C16 (a), DMPC-d67 (b) and DMPC-d67/C16 (20%) (c). The main phase transition temperature of pure C16 as estimated from the (CH2)n resonances is found around 370 K (a) but is lowered significantly to around 320 K in the mixture (c). This value is slightly above the phase transition of the lipids in the mixtures, which itself is elevated compare to pure DMPC (see Fig. S2). The proton spectrum of the mixture is in principle a superposition of lipid and C16 resonances, but one additional peak at 3.25 ppm, labelled ‘A’, appears (c). Comparing spectra in H2O/D2O reveals exchangeable sites such as the NH resonance (d). The peak A is tentatively assigned to the C16 OH proton involved in a H-bond with DMPC (see Fig. 4). (e) 1H-MAS NOESY spectrum of DMPC-d67/C16 (20%) in D2O recorded at 310 K, 10 kHz sample rotation rate and a mixing time of 200 ms. At this temperature and mixing time, resonance A shows a number of cross peaks, with DMPC and C16 supporting our interpretation that A originates from 1-OH or 3-OH in C16 involved in a H-bond to DMPC. The C16 1H resonance assignment is based on solution-state NMR data for C18-ceramide and C16-dihydroceramide [39]. The assignment of DMPC is based on [49].
