Fig. 2.
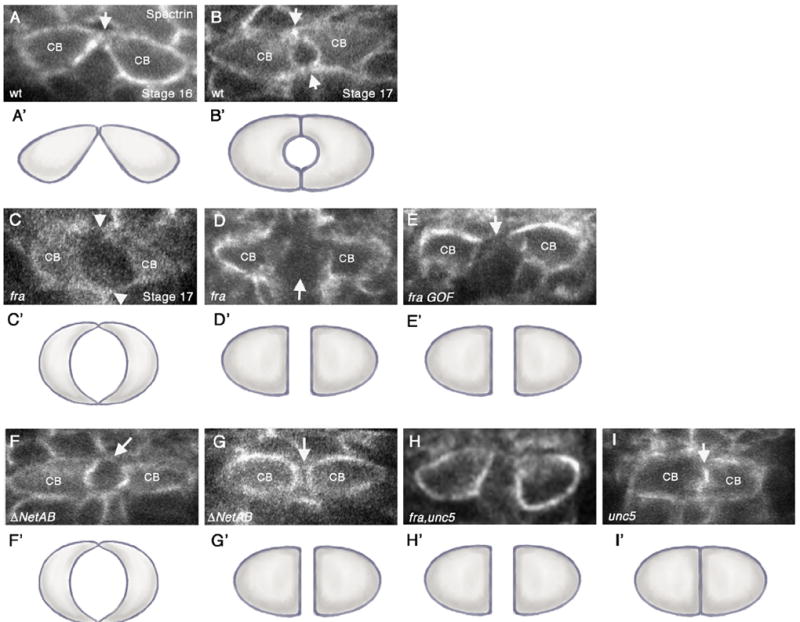
frazzled and Netrin mutants have defects in CB attachment. (A–I) Cross-sections taken through the heart region of the DV (abdominal segments A6–A8) of embryos immunostained with anti-αSpectrin, which outlines the CB membranes. Variances in αSpectrin staining between panels do not necessarily reflect differences in αSpectrin localization but rather in image processing. Except for panel (A), all embryos shown are at stage 17. (A’–I’) are representative cartoons showing the shape of the CBs based on the cross-sections in the corresponding figures. (A, B) wild type (wt) embryos. (A) In early stage 16 wt embryos, contralateral CBs initiate contact at their dorsal-most regions (arrow). (B) By stage 17, both dorsal and ventral contacts have formed (arrows), and a lumen can be observed in between. (C). fra3 mutants show a range of lumen defects (C and D). (C) Diminished adhesion domain phenotype where dorsal and ventral contacts appear to form (arrowheads) but are severely diminished compared to wt. In addition, the lumen appears enlarged. (D) Arrested phenotype, where CBs migrate to the dorsal midline, but then fail to undergo further cell shape changes resulting in a large space in between contralateral CBs (arrow). (E) Overexpression of UAS-fra at high levels in CBs with 24B-Gal4 results in an “arrest” phenotype, where the CBs have failed to initiate dorsal contact (arrow). (F, G) In ΔNetAB embryos, the attachment points between CBs are often diminished or absent (arrows) (F) or CBs fail to undergo cell shape changes leading to failure to make contact (arrow) (G). (H) unc5, fra double mutants show an “arrest” phenotype in which the CBs fail to make contact. (I) In unc58 mutants, we observed inappropriate attachment of CBs (arrow) resulting in a no-lumen phenotype.
