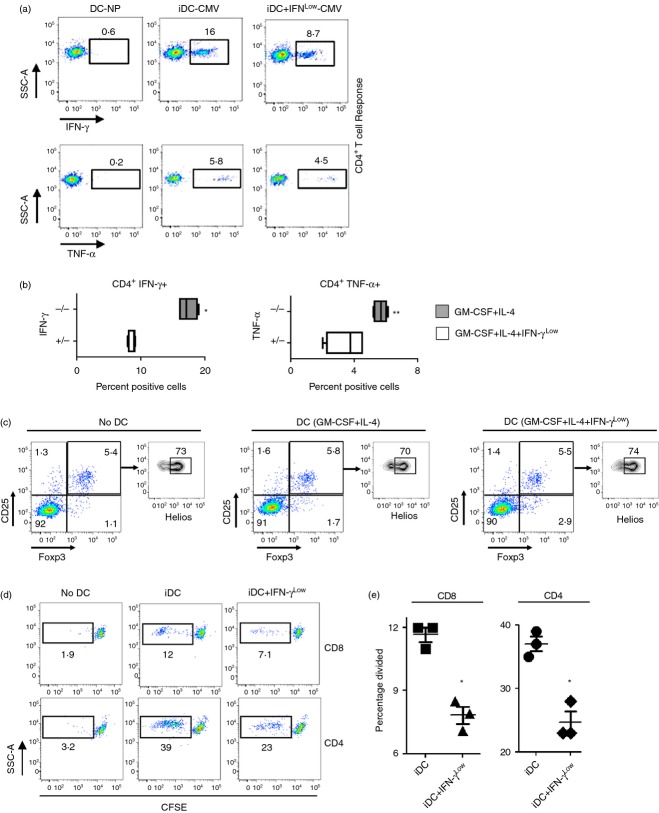
Figure 5.
Monocyte-derived dendritic cells (moDC) exposed to low concentrations of interferon-γ (IFN-γLow) during differentiation show decreased antigen-specific T-cell responsiveness. (a) Representative flow cytometry plots depicting cytomegalovirus-specific autologous CD4+ T-cell responses by intracellular staining for IFN-γ and tumour necrosis factor-α (TNF-α) following priming with IE and pp65 peptide pool pulsed DC differentiated with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) or GM-CSF, IL-4 and 1 U IFN-γ. DC-NP represents co-cultures with DCs not pulsed with peptide. All plots gated on live vivid−, CD3+, CD4+ cells. (b) Quantification of the percentage of live vivid−, CD3+, CD4+, IFN- γ+ and live vivid−, CD3+, CD4+, TNF-α+ cells from co-cultures similar to (a). *P < 0·05, **P < 0·10 compared with co-cultures with moDC grown with GM-CSF, IL-4 and IFN-γLow (+/−). Data are representative of three separate experiments in different healthy donors. (c) Autologous peripheral blood lymphocytes were co-cultured with moDC differentiated with GM-CSF and IL-4 or GM-CSF, IL-4 and IFN-γLow and examined for differences in induced, vivid−, CD3+, CD4+, CD25+, Foxp3+, and Helios−, or naturally occurring, vivid−, CD3+, CD4+, CD25+, Foxp3+, Helios+ T regulatory cells. No DC = freshly thawed lymphocytes without DC co-culture. Data are representative of three separate experiments in different healthy donors. (d) Autologous lymphocytes were labelled with CFSE, co-cultured with moDC described above (c), and examined for CFSE dilution (left panel). (e) Quantification of the percentage of CD4+ and CD8+ T cells that have divided based on CFSE dilution from (d) left panel. *P < 0·05 compared with co-cutlures with immature DC (iDC), two-tailed Student's t-test.