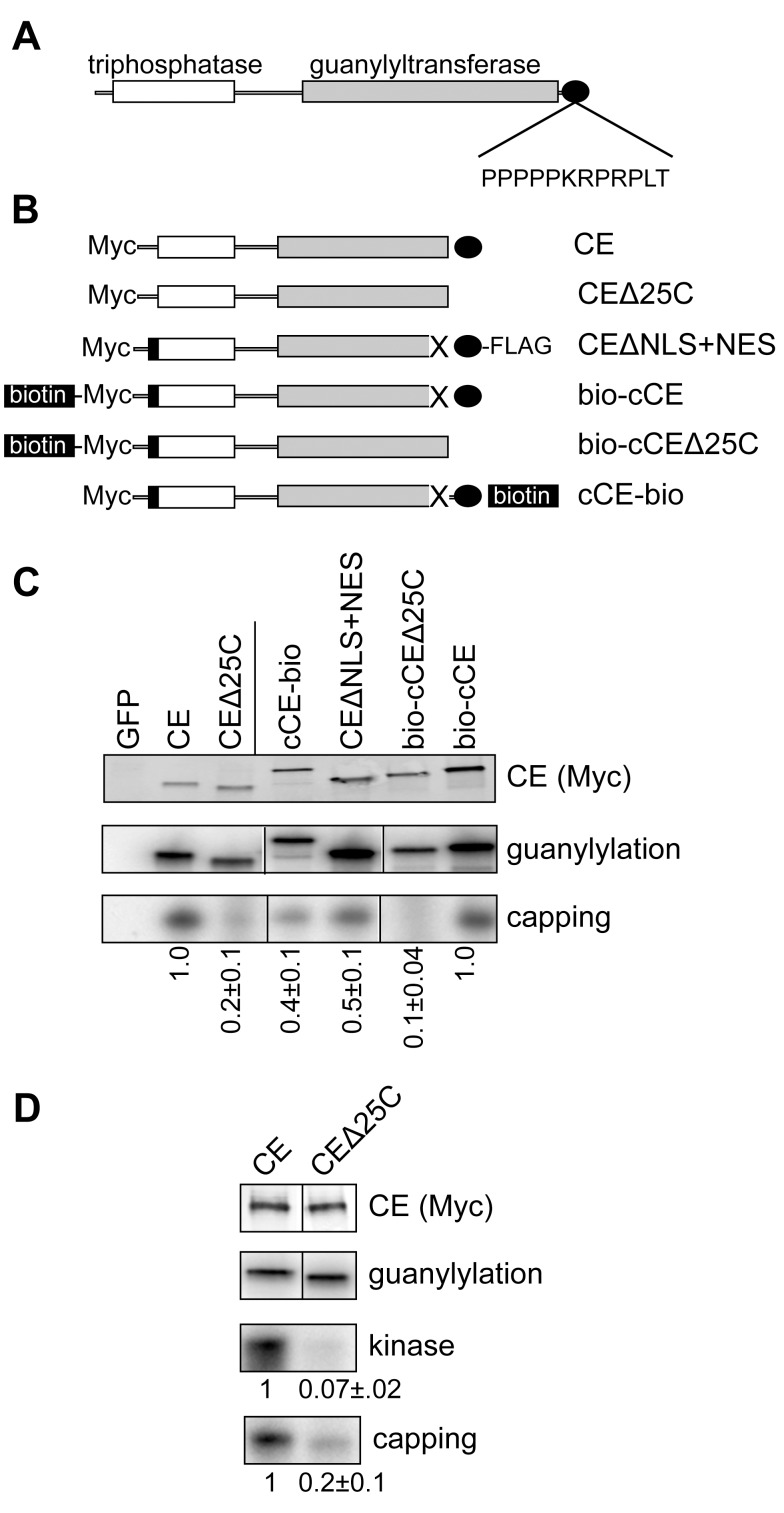
Figure 1. The capping enzyme C-terminus participates in assembly of the cytoplasmic capping complex.
(A) The organization of vertebrate CE is shown with the N-terminal triphosphatase domain indicated in white, the guanylyltransferase domain indicated in grey, and the proline-rich C-terminus indicated by the black oval. The proximity of the proline-rich sequence to the C-terminus is indicated by the sequence of the last 12 amino acids of human CE. (B) The forms of CE used to analyze the impact of C-terminal modifications is shown. In the nomenclature used here CE corresponds to full-length protein with an N-terminal Myc tag. CEΔ25C is the same protein missing the C-terminal 25 amino acids. This deletion includes the NLS. CEΔNLS+NES was described in Otsuka and colleagues [11] and consists of Myc-tagged CE with the NLS deleted (X), a C-terminal FLAG tag and the HIV Rev NES (black box). In bio-cCE the NLS is deleted and a sequence that is biotinylated in vivo is added upstream of Myc tag and Rev NES. In bio-cCEΔ25C the C-terminal 25 amino acids of this protein were deleted. cCE-bio is similar to CEΔNLS+NES except that the C-terminal FLAG tag is replaced with the biotinylation sequence. (C) The plasmids shown in (B) or a plasmid expressing Myc-tagged GFP were transfected into HEK293 cells. GFP or CE and its associated proteins were recovered with anti-Myc beads (CE, CEΔ25C, cCE-bio, and CEΔNLS+NES) or streptavidin beads (bio-cCE and bio-cCEΔ25C). The recovered protein was incubated with α-[32P]GTP and analyzed for the covalent binding of [32P]GMP (guanylylation) [11], and for [32P]GMP labeling of 5′-monophosphate RNA (capping). The products were separated by denaturing gel electrophoresis and visualized by autoradiography. The amount of capping activity relative to guanylylation activity is shown at the bottom of the figure, with results normalized to either CE or to bio-cCE as indicated by the vertical line. (D) The experiment in (C) was repeated with an additional assay for 5′-kinase activity. The vertical line in the Western blot and guanylylation assay is to indicate that CE and CEΔ25C were separated by empty lanes on each of these gels. In (C and D) the mean ± standard deviation (n = 3) for recovered kinase and capping activity normalized to guanylylation activity is shown beneath each autoradiogram. In each case comparison to matching controls yielded p-value<0.05 by Student's t test.