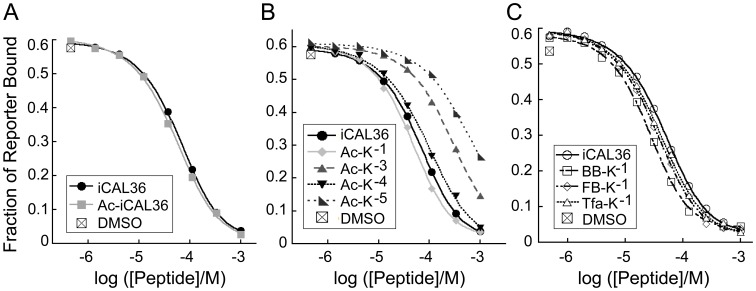
Figure 2. Fluorescence polarization binding studies of modified peptides.
Representative FP isotherms are shown for the displacement of CALP:reporter binding by (A) Ac-iCAL36, (B) scaffold peptides containing acetylated lysine substitutions at each non-motif position, or (C) peptides containing halogenated substituents at the P−1 position (C). In each panel, iCAL36 displacement is also shown for reference. A fluoresceinated iCAL36 peptide (F*-iCAL36) was used as the reporter for all measurements. For illustrative purposes, a logistic curve-fit is shown, but K I values and fractional reporter occupancy values were determined by least-squares fitting of the complete binding equation. Summary values of independent experiments (n = 3) are reported in Table 1.