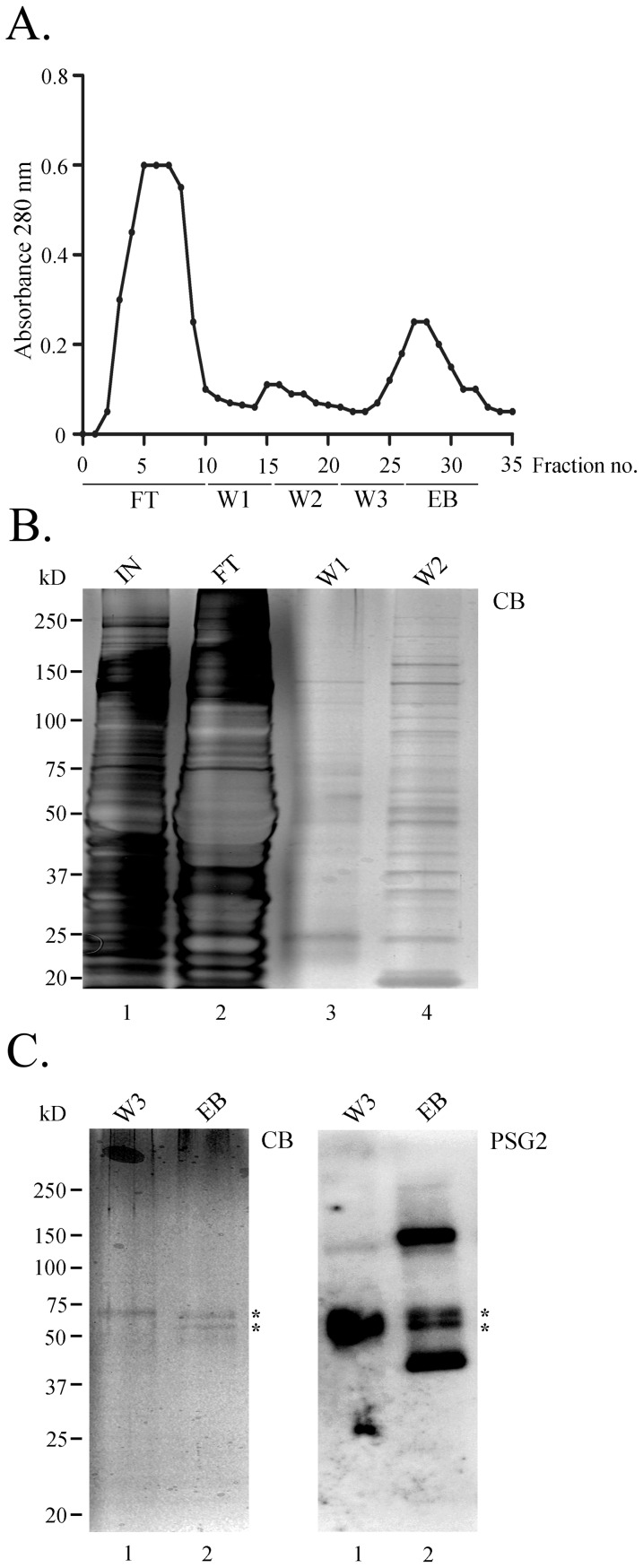
Figure 2. PSG2-immunoaffinity column purification of tyrosine-sulfated proteins from cow RPE.
(A). The elution profile was monitored by following absorbance at 280 nm. Following loading, the column was washed with buffers W1, W2, and W3. Elution was performed in buffer W3 containing 4 mM sulfated pentapeptide (EB). (B). Twenty-six microliter aliquots from input (IN), flow-through (FT), wash 1 (W1), and wash 2 (W2) were fractionated by SDS-PAGE, and proteins were visualized by staining with Coomassie blue dye. (C). Left, SDS-PAGE of 26 µL of wash 3 (W3) and eluted samples (EB) from the immunoaffinity column stained with Coomassie blue (CB) and right, immunoblotted with PSG2. Asterisks indicate the bands that were prominent on Coomassie blue-stained gel (CB) and were also recognized by PSG2 as tyrosine-sulfated.