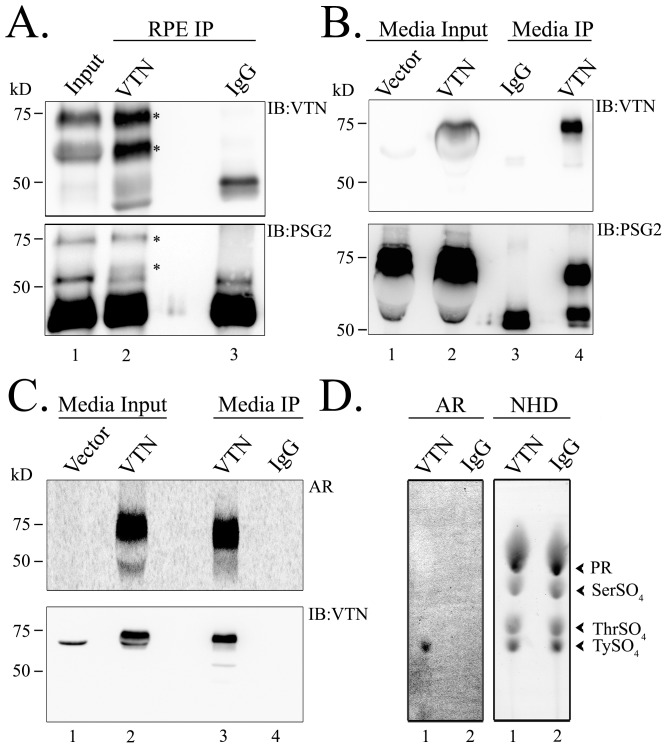
Figure 3. Native human RPE vitronectin is tyrosine-sulfated.
Vitronectin was immunoprecipitated from 500 µg human RPE lysates using anti-VTN antibody (lane 2) or mouse IgG (lane 3). Immunoprecipitants were fractionated by SDS-PAGE, transferred, and immunoblotted using anti-VTN antibody or anti-sulfotyrosine PSG2 antibody. Immunoprecipitation and western blots were repeated 3 independent times using biologically different human RPE samples. (B). Ectopically-expressed vitronectin is tyrosine-sulfated. Recombinant VTN or empty vector (pcDNA3.1) were transfected into HEK 293T cells and immunoprecipitated from conditioned media using anti-VTN antibody (lane 4) or mouse IgG (lane 3). Immunoprecipitants were electrophoresed and immunoblotted using anti-VTN antibody or anti-sulfotyrosine PSG2 antibody. Immunoprecipitation and western blots were repeated 3 independent times after independent VTN transfections. (C). 35S-metabolic labeling of recombinant vitronectin in vitro. Vitronectin-transfectants were radiolabelled with 35Sulfate. Following radiolabeling, vitronectin was immunoprecipitated and blots were either subjected to autoradiography (AR) or immunoblotted with anti-VTN antibody. (D). Radiolabeled vitronectin bands were excised from the membrane along with equivalent areas from mouse IgG immunoprecipitants, and alkaline hydrolysis was performed. The samples were then spiked with sulfo-amino standards tyrosine sulfate, threonine sulfate, and serine sulfate, and subjected to thin layer electrophoresis (TLE) on cellulose plates. Following TLE analysis, sulfo-amino standards (NHD) were visualized either by spraying with Ninhydrin or autoradiography (AR). TLE experiments were repeated at least three independent times.