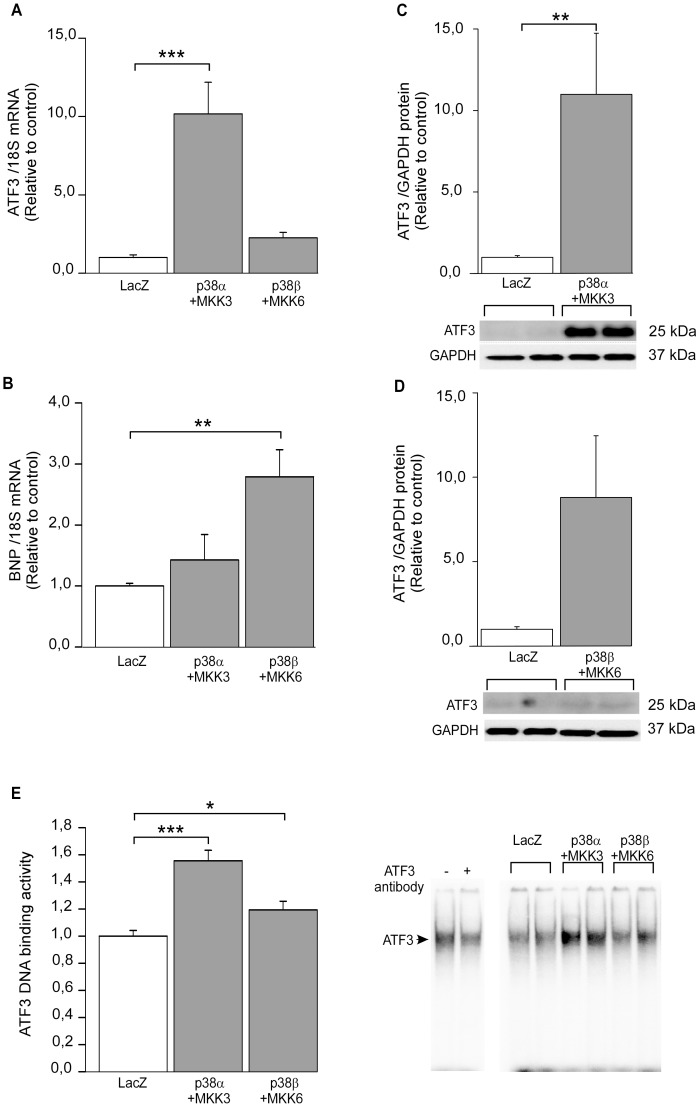
Figure 6. p38α MAPK regulates ATF3 activity.
Cultured cardiomyocytes were transduced with recombinant adenoviruses WT p38α, WT p38β, MKK3b and/or MKK6b for 24 hours at the virus amount of 4 MOI (2+2 MOI in combinations). RT-qPCR with cDNA derived from mRNA of neonatal rat cardiomyocyte cell cultures transduced with recombinant adenovirus combinations WT p38α+MKK3b, WT p38β+MKK6b or control virus LacZ (A–B). ATF3 (A) or BNP (B) mRNA levels were normalized to 18S quantified from the same samples and the mRNA levels are presented relative to LacZ control cells. The results represent mean ± SEM (n = 6–8) from 4 independent experiments. Western blot analysis of cell lysate derived from NRCM cultures transduced with WT p38α and MKK3b (C) or WT p38β and MKK6b (D) recombinant adenoviruses. ATF3 and GAPDH protein levels were detected by Western blotting and representative Western blots are shown. ATF3 protein levels were normalized with GAPDH levels and are presented relative to LacZ control. Bar graphs represent mean ± SEM (n = 6) from 3 independent experiments. EMSA of nuclear protein from adenovirus–transduced cultured cardiomyocytes (E). ATF3 antibody (2 µl) causes supershift reaction (representative blot is shown) and ATF3 binding activity in response to WT p38α+MKK3b and WT p38β+MKK6b is presented as bar graphs (mean ± SEM, n = 12–13 from 3 independent experiments) and representative blot. * P<0.05; **P<0.01; *** P<0.001.