Figure 1.
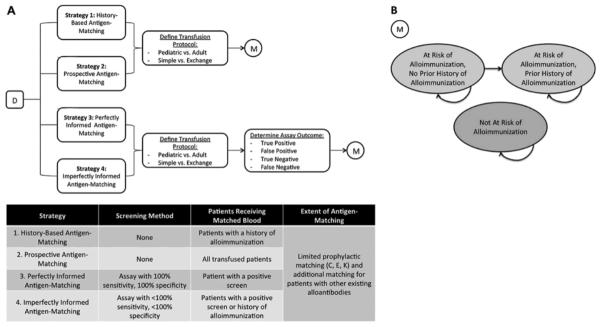
(A). A Markov-based decision tree (D) model was constructed to evaluate alloimmunization events and costs associated with four alternative strategies of antigen-matching among chronically transfusion patients with SCD. Strategy 1, “History-Based Antigen-Matching,” involved providing blood matched for CEK and any other developed alloantibodies only to patients with a history of alloimmunization. Strategy 2 characterized “Prospective Antigen-Matching,” where all transfused patients received prophylactically C, E, K matched blood. Strategy 3, “Perfectly Informed Antigen-Matching,” was based upon a hypothetical assay with 100% sensitivity and 100% specificity. Patients screening positive would prophylactically receive C, E, K antigen-matched blood. Strategy 4 characterized “Imperfectly Informed Antigen-Matching,” where a screening assay with <100% sensitivity and <100% specificity was used to determine which patients prophylactically received C, E, K matched blood. Patients who had not been identified by the imperfect test but developed alloantibodies would also receive antigen-matched blood after the initial alloimmunization event. Outcomes were evaluated for a cohort of initially transfusion-naïve patients with SCD. A series of chance nodes were used to distinguish between patients with different health-related and financial characteristics. For each strategy evaluated, possible transfusion protocols (Pediatric Simple, Pediatric Exchange, Adult Simple, or Adult Exchange) were distinguished. Each protocol was associated with a different number of units transfused and thus a different annual alloimmunization rate. For Strategies 3 and 4, chance nodes were used to distinguish between alternative assay outcomes (True Positive, False Positive, True Negative, or False Negative). (B) For each path along the decision tree, a Markov model (M) was used to track outcomes over a series of 1 year cycles of chronic transfusion therapy. The model consisted of three nodes, “At Risk of Alloimmunization, No Prior History of Alloimmunization,” “At Risk of Alloimmunization, Prior History of Alloimmunization,” and “Not At Risk of Alloimmunization.” At the beginning of the simulation, the patients identified as “at-risk” occupied the first node (At Risk of Alloimmunization, No Prior History of Alloimmunization), while all patients not at risk occupied the third node (Not At Risk of Alloimmunization). Transitions between nodes, as denoted by arrows, could occur between each cycle.
