Abstract
Organosolv pretreatment was used to improve solid-state anaerobic digestion (SSAD) for methane production from three different lignocellulosic substrates (hardwood elm, softwood pine, and agricultural waste rice straw). Pretreatments were conducted at 150 and 180°C for 30 and 60 min using 75% ethanol solution as an organic solvent with addition of sulfuric acid as a catalyst. The statistical analyses showed that pretreatment temperature was the significant factor affecting methane production. Optimum temperature was 180°C for elmwood while it was 150°C for both pinewood and rice straw. Maximum methane production was 152.7, 93.7, and 71.4 liter per kg carbohydrates (CH), which showed up to 32, 73, and 84% enhancement for rice straw, elmwood, and pinewood, respectively, compared to those from the untreated substrates. An inverse relationship between the total methane yield and the lignin content of the substrates was observed. Kinetic analysis of the methane production showed that the process followed a first-order model for all untreated and pretreated lignocelluloses.
1. Introduction
Worldwide concerns about the limitations of fossil resources, rising crude oil prices, and greenhouse gas (GHG) emissions have led researchers to seek alternative clean and renewable energy sources, for example, biofuels [1]. Lignocellulosic materials are abundant and renewable feedstocks that have recently been considered for the production of biofuels [2–6]. Compared to liquid biofuels, biogas has been shown to have far better performance with respect to both agricultural land area efficiency and life cycle assessments [7].
Biogas, produced during anaerobic digestion (AD) processes, can be used as a versatile source of energy to produce heat and electricity, either separate or combined, and to propel vehicles. The production of biogas offers other advantages, such as controlling organic waste, reducing greenhouse gas emissions, and producing another economically viable fertilizer [8, 9]. AD processes are classified into liquid anaerobic digestion (LAD) and solid-state anaerobic digestion (SSAD), based on the solid content [10]. LAD operates at a total solid (TS) content of less than 15%, while SSAD is generally called for a TS content of higher than 15% [11]. Smaller specific reactor volume, fewer moving parts, lower energy input for heating, easier handling of the end product, and lower parasitic energy loss are the main advantages of SSAD in comparison with LAD [11–13]. SSAD is specially required with lignocellulosic feedstocks, such as agricultural residues with low moisture content [11, 14]. However, the anaerobic digestion of lignocelluloses is limited by the rate of hydrolysis due to their recalcitrant structure [15]. Therefore, an additional pretreatment process is essential to improve their digestibility [15, 16].
Although different factors, for example, the crystallinity of cellulose and the accessible surface area, may play important roles in the bioconversion of lignocelluloses, the presence of lignin is apparently the most important factor affecting biodegradability [17–20]. The lignin-carbohydrate matrix limits the digestibility of lignocelluloses since lignin is a hydrophobic polymer that forms a cross-linked network among the carbohydrates. This network is highly resistant to enzymatic and microbial degradations [6, 21]. Hence, the biogas production from lignocelluloses can be improved by a delignification process. Removal of lignin by ethanol is among the most efficient pretreatment techniques in improving bioconversion of lignocelluloses [22, 23]. Furthermore, since lignin is a value added by-product, an additional unique benefit of organosolv pretreatment is unaltered lignin separation [23]. Therefore, using ethanol as an organosolv for pretreatment prior to the AD process has been reported to improve the economy of the process by increasing methane yield and recovery of lignin [24]. To our knowledge, there is no publication in the literature on utilizing organosolv pretreatment prior to SSAD of lignocelluloses.
The main objective of this study was to improve the performance of solid-state anaerobic digestion of three different types of lignocelluloses, that is, elmwood, pinewood, and rice straw, by applying organosolv pretreatments using ethanol under varying conditions. The effects of the pretreatment parameters, that is, temperature and duration time, on the methane yield were determined by solid-state batch anaerobic digestion assays. In addition, the kinetics of the degradation process was investigated for both untreated and pretreated substrates.
2. Material and Methods
2.1. Feedstocks and Inoculum
Elm, a hardwood, pine, a softwood, and rice straw, an agricultural waste, were used as substrates for biogas production. Elmwood and pinewood were obtained from the forest of Isfahan University of Technology (Isfahan, Iran), and rice straw (Sazandegi cultivar, Isfahan, Iran) was sourced from a field in Lenjan Province, Iran. Both elmwood and pinewood were debarked, cut into smaller pieces, and milled to obtain chips of less than 2 cm. The wood chips and the rice straw were partly ball-milled and screened to achieve powder with particle sizes between 295 and 833 μm (20–80 mesh). The screened substrates were then stored in airtight plastic bags at room temperature until use.
Effluent of a 7000 m3 mesophilic anaerobic digester (Isfahan Municipal Sewage Treatment, Isfahan, Iran) was used as inoculum for the batch digestion assays. Due to its low TS content, the inoculum was centrifuged at 4500 rpm for 30 min to obtain the desirable TS content for the SSAD. The supernatant was discharged, and the remaining sludge was mixed to obtain a homogenous inoculum for SSAD. The inoculum was kept at 37°C for one week for stabilization.
2.2. Organosolv Pretreatment
Ethanol as an organic solvent together with sulfuric acid as catalyst was used for the pretreatments. A predetermined amount of each feedstock was mixed with 75% (v/v) aqueous ethanol solution supplemented with 1% w/w (based on dry mass) sulfuric acid to obtain a solid-to-liquid ratio of 1 : 8 (based on dry mass). The pretreatments were carried out in a 500 mL high-pressure stainless steel batch reactor [25]. After loading the substrate and the acidic ethanol mixture, the reactor was heated at a rate of 3°C/min to the desired temperature, that is, 150 or 180°C, and this temperature was held for 30 or 60 min. Then, the reactor was cooled in an ice bath. Afterwards, the pretreated materials were removed, washed three times with 100 mL aqueous ethanol (75% v/v, 60°C), and left overnight to air dry [24, 26]. The pretreated materials were stored in airtight plastic bags at room temperature until use.
2.3. Solid-State Anaerobic Digestion (SSAD) and Modeling
The untreated and pretreated elmwood, pinewood, and rice straw (1 g dry mass) were mixed with a predetermined amount of inoculum and deionized water to achieve a feed-to-inoculum ratio (F/I) (based on volatile solids (VS) content) of 3 and initial TS content of 21%. Sealable 118 mL glass reactors were used for the anaerobic digestion assays. Anaerobic conditions were provided by purging the reactors with nitrogen gas for about 2 min, and the reactors were then incubated in a convection oven at mesophilic conditions (39 ± 1°C) for 55 days [27]. Inoculum (without adding any substrate) was evaluated as a blank to determine the inoculum's methane production. All digestion assays were run in duplicate. Gas samples were taken and analyzed for produced biogas volume and composition in every 3 days during the first 9 days of the experimental period and then in every 5 or 6 days until 55 days.
The kinetics of the anaerobic digestion process was also evaluated using a first-order kinetic model (1). The first-order kinetic model was linearized as shown in (2) [28]:
| (1) |
| (2) |
where t (day) is time and M u and M t (L·kg−1CH) are methane yields obtained in 55 days and t days, respectively, and k is the specific rate constant.
2.4. Analytical Methods
Total solid (TS) and volatile solid (VS) contents of the feedstocks and inoculum were measured by drying the samples at 105°C followed by heating the dried residues at 575°C to a constant weight [17]. The untreated and pretreated samples were analyzed for lignin and hemicellulose contents according to the methods presented by Sluiter et al. [29] and Yang et al. [30], respectively. The cellulose content was calculated as the remaining TS, based on an extractive-free basis, assuming that ash, hemicellulose, lignin, and cellulose are the only components of the entire biomass.
Methane and carbon dioxide produced during the anaerobic digestions were analyzed by a gas chromatograph (Sp-3420A, TCD detector, Beijing Beifen Ruili Analytical Instrument Co., China) equipped with a packed column (3 m length and 3 mm internal diameter, stainless steel, Porapak Q column, Chrompack, Germany). The carrier gas was nitrogen at a flow rate of 45 mL/min. The column, injector, and detector temperatures were 40, 100, and 150°C, respectively. A pressure-tight syringe (VICI, Precision Sampling, Inc., USA) with a volume of 250 μL was used for gas sampling and injection, enabling taking of gas samples at the bioreactors' actual pressure. Excess gas was released through a needle after each gas sampling to avoid overpressure built-up in the bottles.
All biogas yields were presented at standard conditions.
2.5. Statistical Analysis
Analysis of variance (ANOVA) using Minitab software v. 15 was performed to compare confidence intervals and significance between treatments. The factors were considered significant when the probability (P value) was less than 0.05.
3. Results and Discussion
3.1. Characterization of Inoculum
The inoculum obtained from the industrial biogas plant contained 5.7 and 2.7% TS and VS, respectively (Table 1). In order to achieve a TS content of 21% in SSAD, the inoculum was centrifuged [31] to reach TS and VS contents of 11.7% and 5.3%, respectively (Table 1).
Table 1.
Composition analyses of the inoculum as well as the untreated versus pretreated feedstocks.
| Samples | Pretreatment | TS content (%) | VS content (%) | Total lignin∗ (%) | Hemicellulose (%) | Cellulose (%) |
|---|---|---|---|---|---|---|
| Inoculum | — | 5.7 | 2.7 | ND | ND | ND |
| Centrifuged | 11.7 | 5.3 | ND | ND | ND | |
|
| ||||||
| Elmwood | Untreated | 95.5 | 94.5 | 26.2 | 26.3 | 46.4 |
| 150°C, 0.5 h | 95.5 | 94.1 | 25.1 | 23.4 | 50.0 | |
| 150°C, 1 h | 95.5 | 93.8 | 23.4 | 21.5 | 53.3 | |
| 180°C, 0.5 h | 96.3 | 94.4 | 20.4 | 21.9 | 55.7 | |
| 180°C, 1 h | 94.9 | 93.6 | 19.1 | 21.3 | 58.1 | |
|
| ||||||
| Pinewood | Untreated | 95.1 | 95.2 | 26.8 | 28.0 | 44.5 |
| 150°C, 0.5 h | 95.3 | 94.6 | 27.8 | 20.2 | 51.3 | |
| 150°C, 1 h | 95.9 | 95.1 | 26.5 | 21.3 | 51.4 | |
| 180°C, 0.5 h | 96.5 | 95.5 | 22.1 | 18.5 | 58.4 | |
| 180°C, 1 h | 96.9 | 95.8 | 21.1 | 16.9 | 60.8 | |
|
| ||||||
| Rice straw | Untreated | 95.4 | 83.9 | 17.1 | 50.1 | 21.5 |
| 150°C, 0.5 h | 95.6 | 83.8 | 12.2 | 45.6 | 29.9 | |
| 150°C, 1 h | 95.7 | 83.6 | 13.4 | 45.3 | 28.7 | |
| 180°C, 0.5 h | 95.9 | 86.2 | 11.4 | 42.3 | 36.2 | |
| 180°C, 1 h | 96.0 | 84.7 | 10.6 | 42.2 | 35.3 | |
ND = not determined.
∗Sum of acid soluble lignin (ASL) and acid insoluble lignin (AIL) contents.
3.2. The Effect of Different Pretreatment Conditions on the Composition of Substrates
Elmwood, pinewood, and rice straw were subjected to organosolv pretreatment using ethanol prior to anaerobic digestion in order to improve the yield of biogas production. The untreated and pretreated materials were characterized, according to their TS, VS, lignin, cellulose, and hemicellulose contents, and results are summarized in Table 1.
Total lignin contents of untreated elmwood and pinewood were 26.2 and 26.8%, respectively, which was much higher than that of untreated rice straw (17.1%).
The various components of the materials were differently affected by the pretreatments. Depending on the pretreatment conditions, the lignin contents were reduced by 4–27% for elmwood, by 1–21% for pinewood, and by 21–37% for rice straw. Increasing the severity of the pretreatment generally resulted in higher lignin removal. A relatively high portion of straw's lignin (37.7%) was removed through pretreatment at 180°C for 60 min, resulting in a pretreated straw with carbohydrate content of over 77% of TS. On the other hand, the organosolv pretreatment of elmwood and pinewood, at 180°C for 60 min, resulted in 27% and 21% lignin removal, respectively, with corresponding CH contents of 72.7% and 72.5% of TS, respectively. In addition to delignification, parts of hemicelluloses were also removed due to the pretreatments. Higher hemicellulose removal was obtained in pretreated pinewoods (28–40%), compared to that in elmwood (11–19%) or straw (9–16%).
3.3. Biogas Production
Organosolv pretreatments in four different conditions were performed on the three different lignocellulosic materials, and the methane yields of the pretreated and untreated materials were then measured through batch SSAD assays. The accumulated methane productions obtained during 55 days of digestion from the untreated and pretreated materials are shown in Figure 1.
Figure 1.
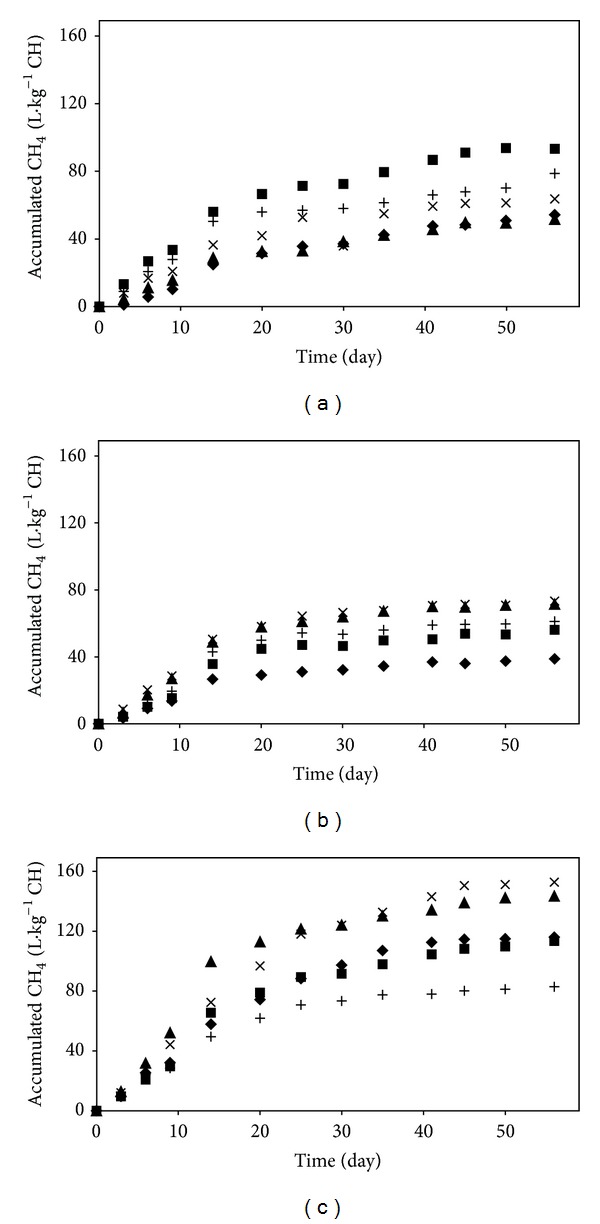
Accumulated methane production from SSAD of untreated and pretreated (a) elmwood, (b) pinewood, and (c) rice straw in different pretreatment conditions. The symbols represent the untreated substrates (◆), the substrates pretreated at 150°C for 0.5 h (▲), at 150°C for 1 h (×), at 180°C for 0.5 h (+), and at 180°C for 1 h (■).
Methane production yields from all of the substrates were generally improved by the pretreatments in all conditions. The highest methane yield of 152.7 L·kg−1CH was obtained from rice straw pretreated at 150°C for 1 h (Table 2). However increasing the pretreatment temperature resulted in a reduced methane yield. This could be due to the inhibitory products which can be formed at high temperature during the pretreatment. In contrast, the highest yield of methane production from pretreated elmwood (93.7 L·kg−1CH) was obtained after pretreatment at 180°C for 1 h; hence, the methane production from elmwood was improved by increasing the severity of the pretreatment. However, the pretreatment of pinewood at 150°C for 0.5 h (the lowest severity) resulted in a methane yield of 71.4 L·kg−1CH, which showed 84% improvement compared to the methane yield from untreated pinewood. Although pretreating pinewood had a remarkable effect on the yield of methane production (i.e., improvements of 45–84%), the statistical analyses using methane yield as response variable showed that neither temperature nor time, with P values of 0.28 and 0.91, respectively, had a significant effect on methane yield in the case of pinewood. In contrast, pretreatment temperature had a significant effect on the methane production from elmwood and rice straw; while being similar to that of pinewood, it was concluded that the effect of pretreatment time on methane production from elmwood, pinewood, and rice straw was not significant (P values of 0.14, 0.91, and 0.27, resp.).
Table 2.
The accumulated methane yields obtained after 55 days of anaerobic digestion from untreated and pretreated lignocellulosic substrates together with the specific rate constants and the regression coefficients calculated from the first-order kinetic model fitting.
| Sample | Pretreated conditions | CH4 (L·kg−1CH) | k (day−1) | r 2 |
|---|---|---|---|---|
| Elmwood | Untreated | 54.2 ± 3.5 | 0.054 | 0.975 |
| 150°C, 0.5 h | 55.4 ± 9.7 | 0.063 | 0.934 | |
| 150°C, 1 h | 63.6 ± 12.3 | 0.066 | 0.914 | |
| 180°C, 0.5 h | 78.7 ± 0.4 | 0.062 | 0.961 | |
| 180°C, 1 h | 93.7 ± 0.9 | 0.097 | 0.937 | |
|
| ||||
| Pinewood | Untreated | 38.7 ± 4.1 | 0.066 | 0.973 |
| 150°C, 0.5 h | 71.4 ± 3.7 | 0.094 | 0.981 | |
| 150°C, 1 h | 63.3 ± 9.3 | 0.073 | 0.933 | |
| 180°C, 0.5 h | 61.1 ± 4.4 | 0.080 | 0.979 | |
| 180°C, 1 h | 56.0 ± 8.5 | 0.065 | 0.962 | |
|
| ||||
| Rice straw | Untreated | 115.9 ± 12.8 | 0.081 | 0.943 |
| 150°C, 0.5 h | 143.3 ± 7.1 | 0.084 | 0.946 | |
| 150°C, 1 h | 152.7 ± 20.2 | 0.088 | 0.918 | |
| 180°C, 0.5 h | 93.8 ± 19.9 | 0.078 | 0.991 | |
| 180°C, 1 h | 113.4 ± 1.6 | 0.068 | 0.984 | |
Among the untreated samples, the highest methane yield, 115.9 L·kg−1CH, was obtained from rice straw, which had the lowest lignin content among the substrates utilized in this study. The digestion of untreated elmwood and pinewood resulted in methane yields of 54.2 and 38.7 L·kg−1CH, respectively. The presence of pores in the structure of hardwoods which facilitate microorganisms' accessibility might be responsible for the higher yield obtained from elmwood in comparison to that from pinewood [32].
3.4. Methane Production Modeling
The fitting of kinetics data on the first-order model for all of the substrates is shown in Table 2, as well as the accumulated methane yields obtained after 55 days of SSAD. The regression coefficients demonstrated that methane production followed the first-order kinetic model (r 2 > 0.91). At the optimum pretreatment conditions for each substrate, that is, 180°C and 1 h, 150°C and 0.5 h, and 150°C and 1 h for elmwood, pinewood, and rice straw, the corresponding k value was at its maximum level, respectively, representing the highest degradation rate for each investigated substrate.
3.5. Relationship between Total Lignin Content and Methane Yield from Lignocellulosic Materials
The effect of lignin content on final methane yield was investigated by comparing methane yield as a function of the materials' lignin content (Figure 2). In line with a previous study [28], an overall inverse relationship between the lignin content of different substrates and the achieved methane yields was observed. However, the low linear regression coefficient of 0.7 confirmed that the content of lignin is not the sole key factor affecting methane yield. The contents of cellulose and hemicellulose, the crystallinity of cellulose, and the accessible surface area may also play important roles affecting methane yields [17–20]. Therefore, further investigations are required to find the specific reason for the observed improvements.
Figure 2.
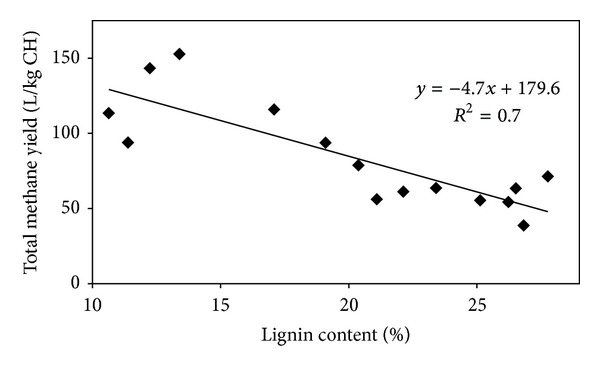
Relationship between lignin content and total methane yield from lignocellulosic substrates (untreated and pretreated elmwood, pinewood, and rice straw).
4. Conclusions
Organosolv pretreatment prior to SSAD was an efficient process for improvement of methane production from different types of lignocellulosic materials; however, its effectiveness greatly depended on the type of lignocelluloses. The pretreatment process was more effective on softwood than on hardwood or agricultural waste. Moreover, hardwood needed more severe conditions to be able to achieve maximum improvement during the subsequent batch digestion assays. Lignin content was among the most important factors negatively affecting the methane production from all of the investigated lignocellulosic substrates.
Acknowledgments
The authors are grateful for financial support from the Region Västra Götaland and the Institute of Biotechnology and Bioengineering, Isfahan University of Technology.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
All experiments and paper preparation were performed by Safoora Mirmohamadsadeghi. The coauthors supervised the experiments and helped with paper preparation.
References
- 1.Deublein D, Steinhauser A. Biogas from Waste and Renewable Resources: An Introduction. Wiley; 2008. [Google Scholar]
- 2.Gnansounou E. Production and use of lignocellulosic bioethanol in Europe: current situation and perspectives. Bioresource Technology. 2010;101(13):4842–4850. doi: 10.1016/j.biortech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Hromádko J, Hromádko J, Miler P, Hönig V, Cindr M. Technologies in second-generation biofuel production. Chemicke Listy. 2010;104(8):784–790. [Google Scholar]
- 4.Sims REH, Mabee W, Saddler JN, Taylor M. An overview of second generation biofuel technologies. Bioresource Technology. 2010;101(6):1570–1580. doi: 10.1016/j.biortech.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 5.Sivakumar G, Vail DR, Xu J, et al. Bioethanol and biodiesel: Alternative liquid fuels for future generations. Engineering in Life Sciences. 2010;10(1):8–18. [Google Scholar]
- 6.Kumar S. Hydrothermal processing of biomass for biofuels. Biofuel Research Journal. 2014;2:p. 43. [Google Scholar]
- 7.Börjesson P, Mattiasson B. Biogas as a resource-efficient vehicle fuel. Trends in Biotechnology. 2008;26(1):7–13. doi: 10.1016/j.tibtech.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Taleghani G, Kia AS. Technical-economical analysis of the Saveh biogas power plant. Renewable Energy. 2005;30(3):441–446. [Google Scholar]
- 9.Balat M, Balat H. Biogas as a renewable energy source—a review. Energy Sources, Part A: Recovery, Utilization and Environmental Effects. 2009;31(14):1280–1293. [Google Scholar]
- 10.Li Y, Park SY, Zhu J. Solid-state anaerobic digestion for methane production from organic waste. Renewable and Sustainable Energy Reviews. 2011;15(1):821–826. [Google Scholar]
- 11.Brown D, Shi J, Li Y. Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresource Technology. 2012;124:379–386. doi: 10.1016/j.biortech.2012.08.051. [DOI] [PubMed] [Google Scholar]
- 12.Guendouz J, Buffière P, Cacho J, Carrère M, Delgenes J. High-solids anaerobic digestion: comparison of three pilot scales. Water Science and Technology. 2008;58(9):1757–1763. doi: 10.2166/wst.2008.521. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y-S, Zheng Y, Yu CW, Dooley TM, Jenkins BM, Vandergheynst JS. Evaluation of high solids alkaline pretreatment of rice straw. Applied Biochemistry and Biotechnology. 2010;162(6):1768–1784. doi: 10.1007/s12010-010-8958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singhania RR, Patel AK, Soccol CR, Pandey A. Recent advances in solid-state fermentation. Biochemical Engineering Journal. 2009;44(1):13–18. [Google Scholar]
- 15.Taherzadeh MJ, Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. International Journal of Molecular Sciences. 2008;9(9):1621–1651. doi: 10.3390/ijms9091621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teghammar A, Karimi K, Sárvári Horváth I, Taherzadeh MJ. Enhanced biogas production from rice straw, triticale straw and softwood spruce by NMMO pretreatment. Biomass and Bioenergy. 2012;36:116–120. [Google Scholar]
- 17.Chang VS, Holtzapple MT. Fundamental factors affecting biomass enzymatic reactivity. Proceedings of the 21st Symposium on Biotechnology for Fuels and Chemicals; 2000; Springer; [DOI] [PubMed] [Google Scholar]
- 18.Laureano-Perez L, Teymouri F, Alizadeh H, Dale BE. Understanding factors that limit enzymatic hydrolysis of biomass: Characterization of pretreated corn stover. Applied Biochemistry and Biotechnology. 2005;124(1–3):1081–1099. doi: 10.1385/abab:124:1-3:1081. [DOI] [PubMed] [Google Scholar]
- 19.Puri VP. Effect of crystallinity and degree of polymerization of cellulose on enzymatic saccharification. Biotechnology and Bioengineering. 1984;26(10):1219–1222. doi: 10.1002/bit.260261010. [DOI] [PubMed] [Google Scholar]
- 20.Koullas DP, Christakopoulos P, Kekos D, Macris BJ, Koukios EG. Correlating the effect of pretreatment on the enzymatic hydrolysis of straw. Biotechnology and Bioengineering. 1992;39(1):113–116. doi: 10.1002/bit.260390116. [DOI] [PubMed] [Google Scholar]
- 21.Poornejad N, Karimi K, Behzad T. Ionic liquid pretreatment of rice straw to enhance saccharification and bioethanol production. Journal of Biomass to Biofuel. 2014;2(1):8–15. [Google Scholar]
- 22.Binod P, Sindhu R, Singhania RR, et al. Bioethanol production from rice straw: an overview. Bioresource Technology. 2010;101(13):4767–4774. doi: 10.1016/j.biortech.2009.10.079. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Cheng K, Liu D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Applied Microbiology and Biotechnology. 2009;82(5):815–827. doi: 10.1007/s00253-009-1883-1. [DOI] [PubMed] [Google Scholar]
- 24.Obama P, Ricochon G, Muniglia L, Brosse N. Combination of enzymatic hydrolysis and ethanol organosolv pretreatments: Effect on lignin structures, delignification yields and cellulose-to-glucose conversion. Bioresource Technology. 2012;112:156–163. doi: 10.1016/j.biortech.2012.02.080. [DOI] [PubMed] [Google Scholar]
- 25.Amiri H, Karimi K, Roodpeyma S. Production of furans from rice straw by single-phase and biphasic systems. Carbohydrate Research. 2010;345(15):2133–2138. doi: 10.1016/j.carres.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Amiri H, Karimi K, Zilouei H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresource Technology. 2014;152:450–456. doi: 10.1016/j.biortech.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Hansen TL, Schmidt JE, Angelidaki I, et al. Method for determination of methane potentials of solid organic waste. Waste Management. 2004;24(4):393–400. doi: 10.1016/j.wasman.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Liew LN, Shi J, Li Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass & Bioenergy. 2012;46:125–132. [Google Scholar]
- 29.Sluiter A, Hames B, Ruiz R, et al. Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure; 2008. [Google Scholar]
- 30.Yang H, Yan R, Chen H, Zheng C, Lee DH, Liang DT. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy and Fuels. 2006;20(1):388–393. [Google Scholar]
- 31.Brown D, Li Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresource Technology. 2013;127:275–280. doi: 10.1016/j.biortech.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 32.Reiterer A, Sinn G, Stanzl-Tschegg SE. Fracture characteristics of different wood species under mode I loading perpendicular to the grain. Materials Science and Engineering A. 2002;332(1-2):29–36. [Google Scholar]


