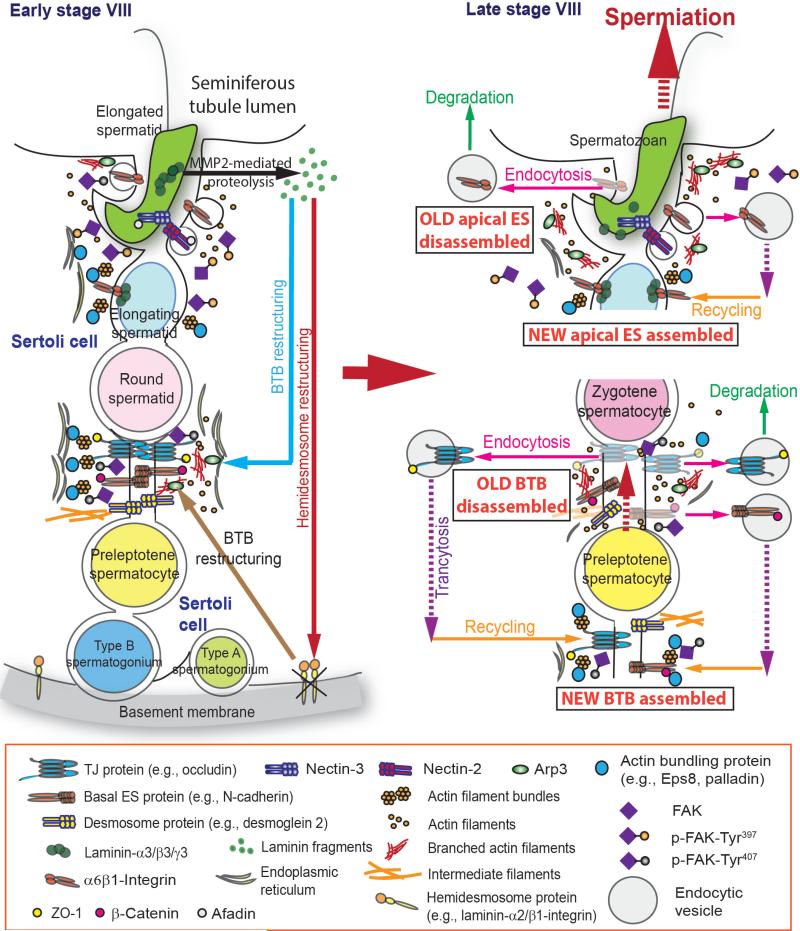
Fig. 3.
A hypothetical model illustrating the physiological role of p-FAK-Tyr397 and p-FAK-Tyr407 in coordinating the apical and the basal ES disruption to facilitate spermiation and BTB restructuring at stage VIII of the epithelial cycle. At stage VIII of the epithelial cycle, spermiation and BTB restructuring that facilitate the release of sperm and the transport of preleptotene spermatocytes across the BTB take place simultaneously, but at opposite ends of the seminiferous epithelium. As detailed in the text, p-FAK-Tyr397 and -Tyr407 play crucial roles in coordinating these cellular events. Shown on the left panel, biologically active fragments of laminin chains released via the action of MMP-2 during the degeneration of apical ES induce BTB restructuring possibly via the action of p-FAK-Tyr407, which likely induces an activation and the recruitment of Arp2/3 protein complex to induce branched actin polymerization, effectively converting actin filaments from a “bundled” to a “branched/de-bundled” configuration, this thus enhances endocytic vesicle-mediated protein trafficking including transcytosis and recycling so that a “new” BTB is assembled at the basal region of the preleptotene spermatocyte being transported across the BTB prior to the disassembly of the “old” BTB above the spermatocyte (see bottom right panel). On the other hand, p-FAK-Tyr397 at the apical ES also recruits the Arp2/3 complex but re-localizes actin bundling proteins Eps8 and palladin away from the apical ES, the net results thus favors the “branched/de-bundled” actin filaments at the apical ES, facilitating endocytic vesicle-mediated protein degradation and/or transcytosis and recycling to assemble “new” apical ES for the newly formed step 8 spermatids at stage VIII of the epithelial cycle. This hypothesis is supported by recent findings in the field as discussed in the text.