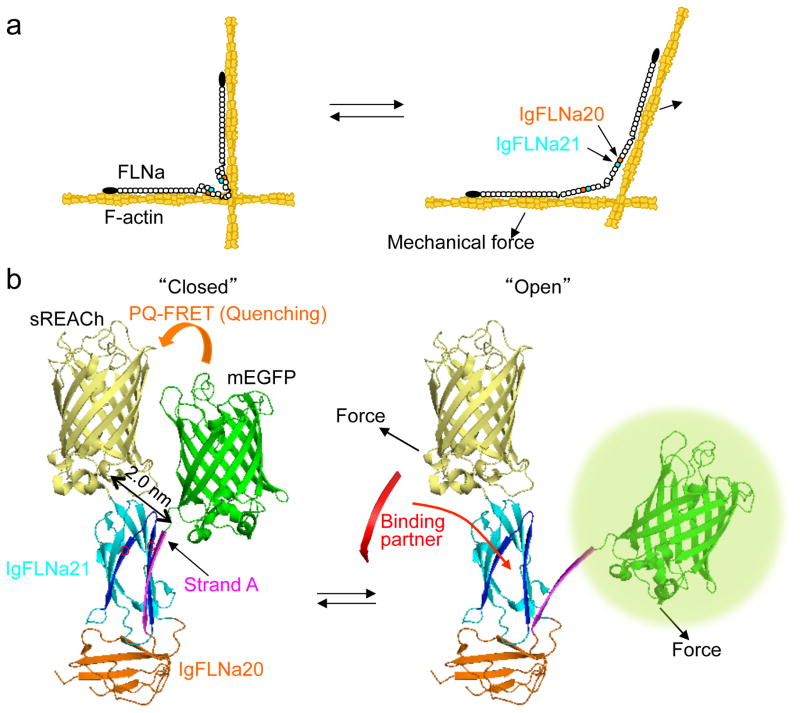
Figure 1. The FLNA repeat 21-conformation sensor.
(a) Model of FLNA crosslinking F-actin showing how its molecular conformation is altered by mechanical forces. FLNA interacts with F-actin through its N-terminal ABD and repeat 10 of rod-1 segment. This posits the rod-2 domain and the self-association site at the vertex of the crosslink where actin filament displacement can linearize the rod 2 domain. (b) Structure of FLNA repeats 20–21 (Protein Data Bank 2J3S) showing how strand A of repeat 20 auto-inhibits the partner binding site on repeat 21 and how mechanical force displaces repeat 20 strand A to expose the 21 partner binding site. The hypothetical location of mEGFP (donor) and sREACh (quencher) on the open and closed conformation of repeats 20–21 is shown. In the non-stressed state (left), the two fluorophores are in close proximity and the fluorescence of mEGFP is quenched by sREACh (or lifetime of mEGFP is shortened). When force opens the cryptic binding site (right), the probes move apart (PQ-FRET efficiency decreases and the fluorescence intensity of mEGFP increases or its lifetime becomes longer).