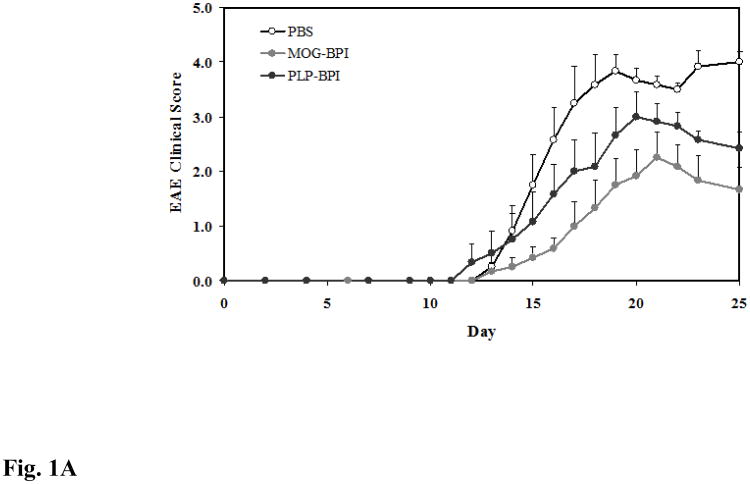
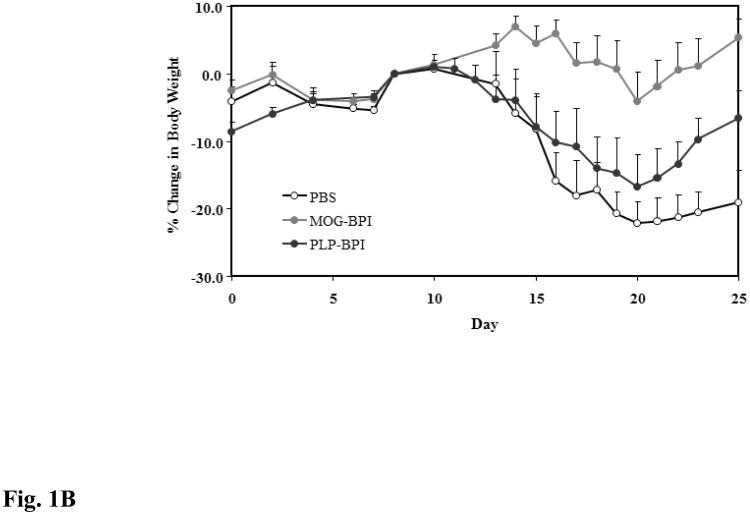
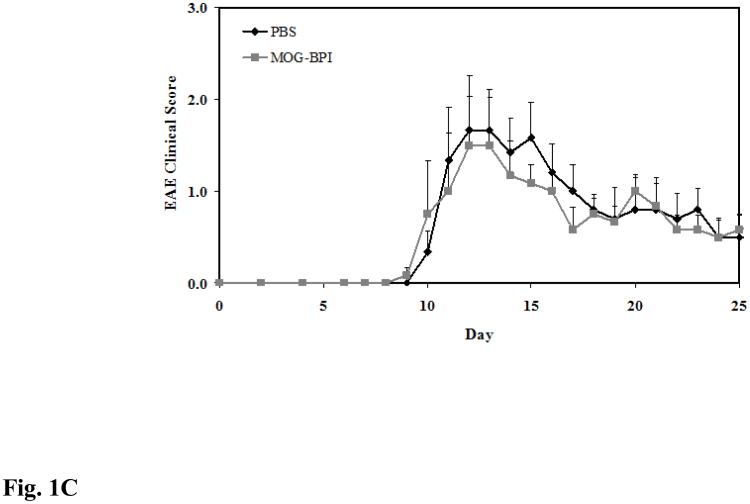
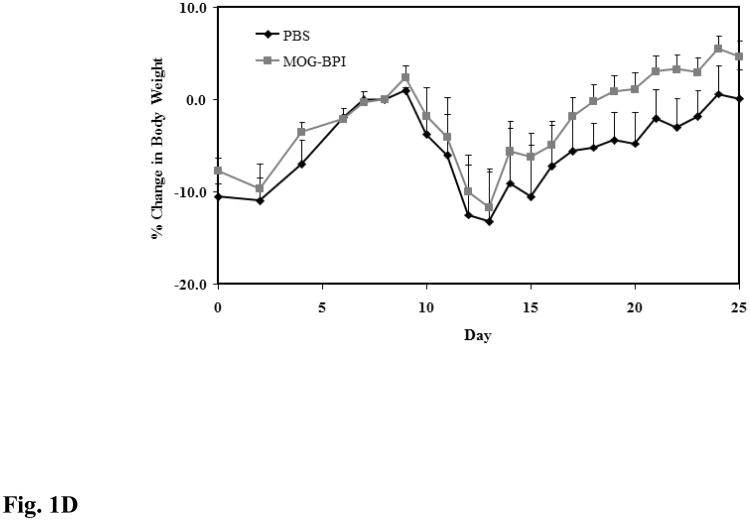
Figure 1.
In vivo cross-reactivity of MOG-BPI and PLP-BPI. PBS (100 μl) and peptides (100 nmol/100 μl) were administered s.c. on days 4, 7, and 10. MOG-BPI- and PLP-BPI-treated mice were compared to PBS-treated mice in the MOG-induced EAE model and efficacy was determined by (A) clinical disease score of EAE, expressed as the mean clinical score ± SEM (n = 6), and (B) percent change in body weight, expressed as the mean % change in body weight ± SEM (n = 6). The efficacy of MOG-BPI in suppressing disease in PLP-induced EAE mice was determined using the (C) clinical disease score of EAE, expressed as the mean clinical score ± SEM (n = 6), and (D) percent change in body weight, expressed as the mean % change in body weight ± SEM (n = 6).