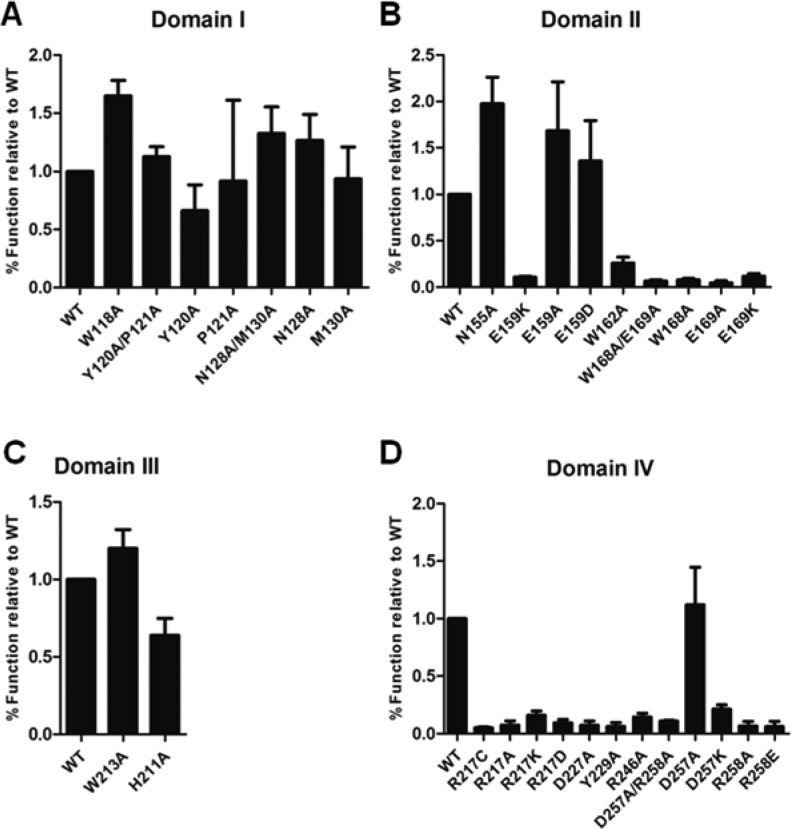
Figure 2.
Substitution of conserved residues in HCCS putative domains modulate synthetase function. Recombinant GST-HCCS variants with substitutions in (A) Domain I, (B) Domain II, (C) Domain III, and (D) Domain IV were coexpressed with cytochrome c in Δccm E. coli. Cells were lysed with BPER reagent, and protein extracts were resolved by SDS-PAGE and transferred to nitrocellulose. Released cytochrome c was detected by heme stain and signal intensity was quantified by densitometry and plotted in GraphPad Prism. Data shown represent the average amount of cytochrome c released by each mutant relative to the level released by WT ± SEM, n = 4.