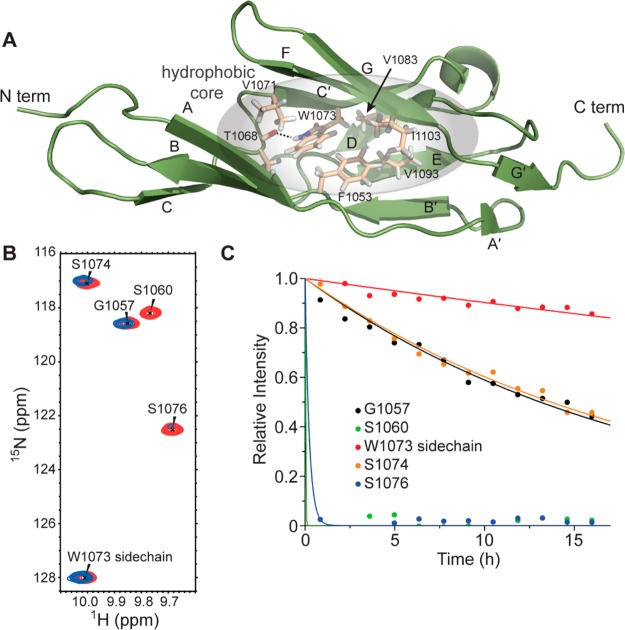
Figure 3.
Hydrophobic core of LigB-12. (A) Structure of LigB-12 showing the residues in the hydrophobic core. (B) A section of the LigB-12 15N,1H NMR spectrum (red) is shown overlaid with a spectrum of the same protein after replacing the solvent with D2O for 12 h (blue). (C) H–D exchange data for the five backbone amide protons shown in panel B. Note the slow exchange of the side chain of W1073.