Figure 4.
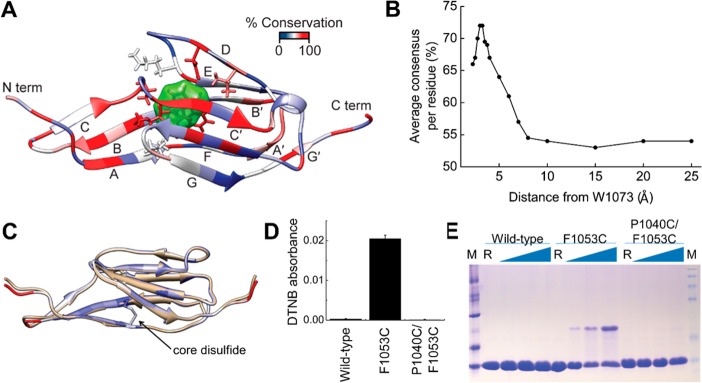
Conservation of Lig Ig-like domains. (A) Residue conservation mapped onto the LigB-12 structure. The core tryptophan, W1073, is shown with a green surface, while atoms for the neighboring residues are also shown in stick representation. (B) The average residue consensus for aligned LigB domains plotted as a function of distance to the core tryptophan (W1073 in LigB-12). (C) The disulfide seen in LigC-2 was transplanted to LigB-12 by making the P1040C/F1053C mutation of LigB-12. Shown is the model of the mutant protein (white) overlaid on the LigB-12 structure (beige). (D) Absorption of the reaction product (TNB2–) at 412 nm after DTNB modification of a free cysteine for wild-type LigB-12 and the F1053C and P1040C/F1053C mutations (n = 3). (E) A nonreducing SDS-PAGE gel was used to detect the formation of interdomain disulfides at increasing temperatures. A significant increase in disulfide-trapped dimers was seen for the single cysteine but not the double cysteine mutant.
