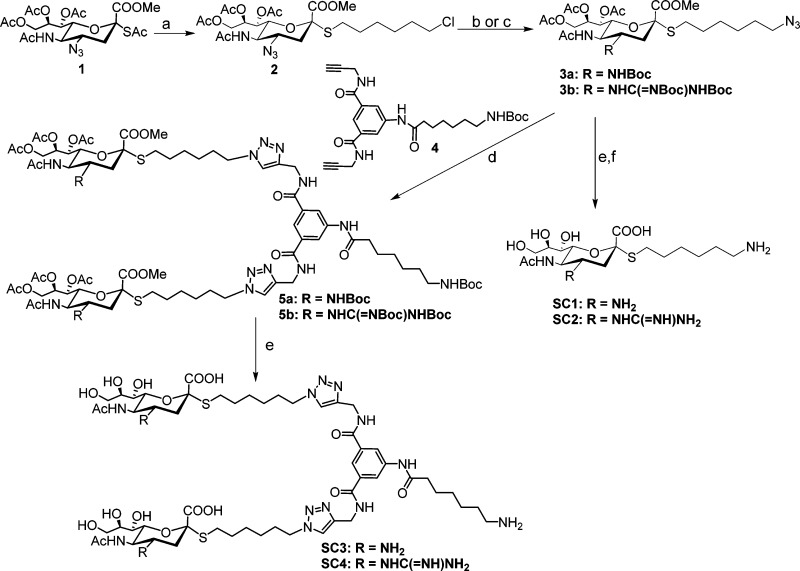
Scheme 1. Reagents and Conditions.
(a) 6-Chlorohexyl 4-methylbenzenesulfonate, DEA, DMF, rt, 4 h. 80% (b) i. PPh3, THF/H2O (1:1), 40 °C, 12 h. ii. (t-Boc)2O, TEA, THF, 60%. iii. NaN3, DMF, 60 °C. 90%. (c) i. PPh3, THF/H2O (1:1), 40 °C, 12 h. ii. 1,3-Bis(tert-butoxycarbonyl)-2-methyl-2-thiopseudourea, TEA, HgCl2, 85%. iii. NaN3, DMF, 60 °C. 93%. (d) Na-L-ascorbate, CuSO4, THF/H2O. 12 h, 60% for 5a; 65 for 5b. (e) i. NaOMe, MeOH. ii. DCM/TFA. iii. NaOH, MeOH, 80% yield for SC3, 70% yield for SC4. (f) H2, Lindlar catalyst, EtOH/H2O, 4 h, 75% yield for SC1, 70% yield for SC2.