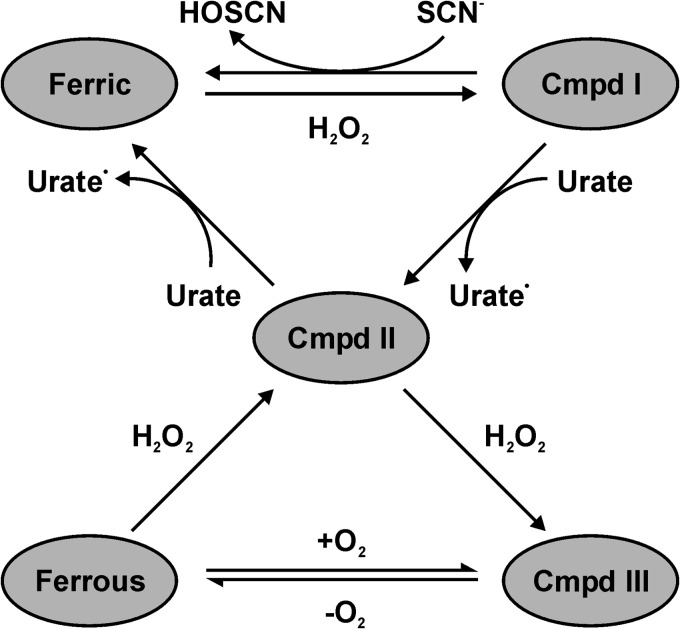
FIGURE 11.
Suggested catalytic mechanism of LPO in an environment where similar concentrations of thiocyanate and urate are present. At low concentrations of hydrogen peroxide, urate will compete with thiocyanate for reaction with compound I (Cmpd I). Which substrate dominates will depend on their relative local concentrations. Urate will also ensure that LPO is not trapped at compound II (Cmpd II), thereby ensuring continual oxidant production by the enzyme. At high concentrations of hydrogen peroxide, LPO will be converted compound III (Cmpd III), whereas its conversion back to compound II relies on its slow release of oxygen (55). Under these conditions, the bactericidal activity of LPO will be inhibited.