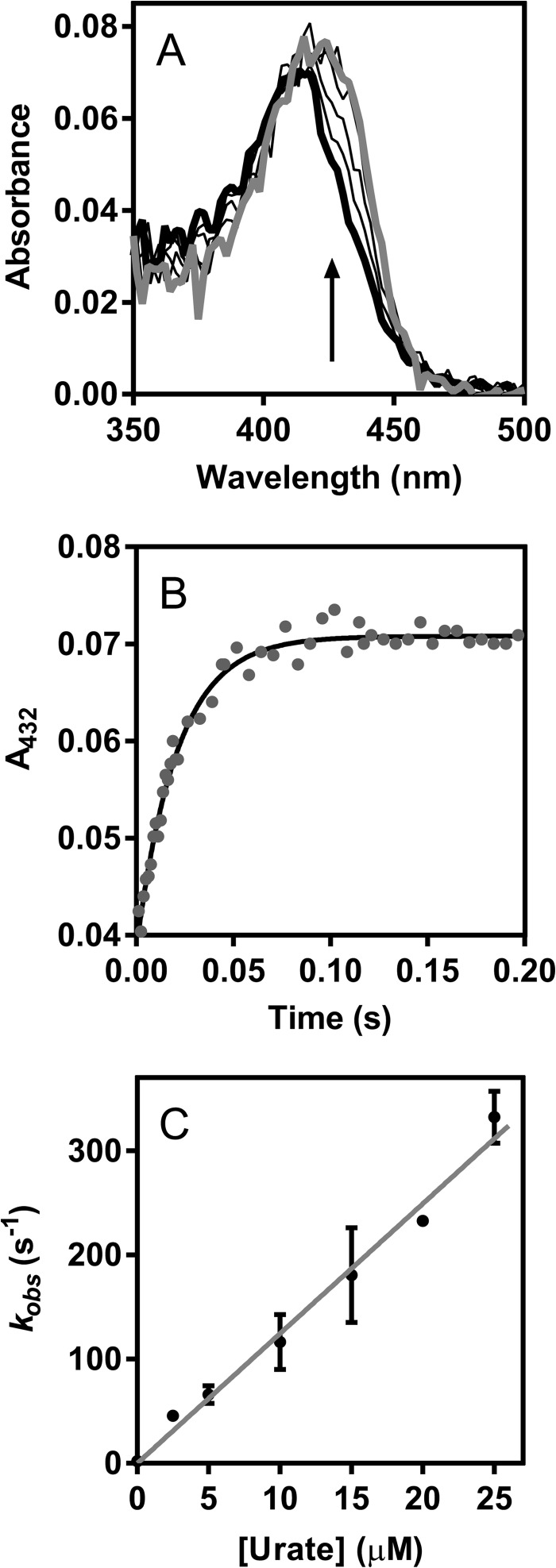
FIGURE 3.
Determination of the rate constant for the reaction of LPO compound I with urate. LPO (1 μm) was premixed with hydrogen peroxide (1 μm) to form compound I at 25 °C in 50 mm phosphate buffer, pH 7.0. After a delay time of 100 ms, urate was added (0–25 μm). All solutions were prepared freshly and kept in the dark to avoid photolysis. A, spectral changes after the addition of urate (2.5 μm) followed between 3 ms (thick black line) and 200 ms (gray line); only representative spectra are shown. B, formation of compound II in A was monitored at 432 nm (gray dots), and the data were fitted by single exponential functions (black line). C, the averaged observed rate constants kobs were plotted against the concentration of urate (black dots). Data are means and standard deviations of at least triplicate measurements. The slope of the linear fit corresponds to the second-order rate constant of the reaction of LPO compound I with urate.