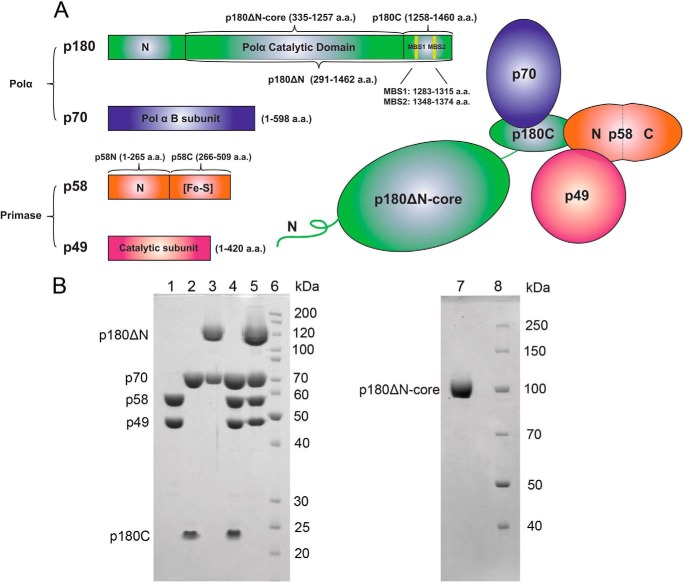
FIGURE 1.
Overall structure of human polα-primase. A, schematic representation of the four-subunit complex, p180 (green), p70 (blue), p58 (orange), and p49 (red). The primary amino acids sequence and the domains of each subunit and various recombinant constructs studied in the current work are shown as stick diagrams on the left. Metal binding motifs in p180 are marked by yellow vertical lines and named two metal binding sites MBS1 and MBS2. B, analysis of the purity of human primase and polα samples. Left panel: lane 1, p49·p58; lane 2, p70·p180C; lane 3, p70·p180ΔN; lane 4, p49·p58·p70·p180C; lane 5, p49·p58·p70·p180ΔN; lane 6, EZ-Run Rec protein ladder (Fisher). Samples were run on 10% SDS-PAGE, and proteins were detected by Coomassie Blue staining. The p180ΔN corresponds to the catalytic subunit with deleted 291 amino acids from the N terminus. Right panel: lane 1, p180ΔN-core, lacking CTD of the catalytic subunit; lane 2, Page Ruler protein ladder (Thermo Scientific). Samples were run on 8% SDS-PAGE and visualized by Coomassie Blue staining.