Abstract
Liver-specific β-catenin knockout (β-Catenin-LKO) mice have revealed an essential role of β-catenin in metabolic zonation where it regulates pericentral gene expression and in initiating liver regeneration (LR) after partial hepatectomy (PH), by regulating expression of Cyclin-D1. However what regulates β-catenin activity in these events remains an enigma. Here, we investigate to what extent β-catenin activation is Wnt-signaling dependent and the potential cell source of Wnts. We studied liver-specific Lrp5/6 KO (Lrp-LKO) mice where Wnt-signaling was abolished in hepatocytes while the β-catenin gene remained intact. Intriguingly, like β-catenin-LKO mice, Lrp-LKO exhibited a defect in metabolic zonation observed as lack of glutamine synthetase (GS), Cyp1a2 and Cyp2e1. Lrp-LKO also displayed a significant delay in initiation of LR due to absence of β-catenin-TCF4 association and lack of Cyclin-D1. To address the source of Wnt proteins in liver, we investigated conditional Wntless (Wls) KO mice, which lacked ability to secrete Wnts from either liver epithelial cells (Wls-LKO), or macrophages including Kupffer cells (Wls-MKO), or endothelial cells (Wls-EKO). While Wls-EKO was embryonic lethal precluding further analysis in adult hepatic homeostasis and growth, Wls-LKO and Wls-MKO were viable but did not show any defect in hepatic zonation. Wls-LKO showed normal initiation of LR, however Wls-MKO showed a significant but temporal deficit in LR that was associated with decreased β-catenin-TCF4 association and diminished Cyclin-D1 expression.
Conclusion
Wnt-signaling is the major upstream effector of β-catenin activity in pericentral hepatocytes and during LR. Hepatocytes, cholangiocytes or macrophages are not the source of Wnts in regulating hepatic zonation. However, Kupffer cells are a major contributing source of Wnt secretion necessary for β-catenin activation during LR.
Keywords: Partial hepatectomy, proliferation, macrophage, glutamine synthetase, Cyclin-D1, non-parenchymal cells, Wntless, Evenness Interrupted
β-Catenin is a transcriptional co-activator that plays a critical role in liver biology. In liver homeostasis, hepatocytes exhibit molecular heterogeneity based on their location within the hepatic lobule, which is known as hepatic zonation. β-catenin is one of the key molecules regulating the zonation pattern. Pericentral hepatocytes express cytoplasmic and nuclear β-catenin in addition to membranous localization. Here, β-catenin regulates the expression of genes such as glutamine synthetase (GS) and certain cytochrome P450 enzymes (CYPs), such as Cyp1a2 and Cyp2e1 (1). In addition, β-catenin signaling is also essential for the initiation of liver regeneration (LR) (2). Liver has the unique capacity to regenerate following partial hepatectomy (PH). During LR a series of cell signaling pathways and cascades are triggered that are tightly regulated. β-Catenin signaling is one such pathway that is activated very early after PH (3). Indeed, others and we have previously shown that liver-specific β-catenin knockout (β-catenin-LKO) that lack this protein in both hepatocytes and cholangiocytes show defective pericentral gene expression and a 24 hour delay in entry of hepatocytes to S-phase after PH that peaks at 72 hours instead of 40 hours (4–6). However what is the upstream regulator of β-catenin in these events remains unknown.
Numerous signaling cascades can activate β-catenin. Canonical Wnt-signaling is the major pathway that induces β-catenin activation (7). Wnt is an extracellular glycoprotein secreted by various types of cells. Binding of Wnt to its cell surface receptor Frizzled and co-receptor low-density lipoprotein related protein-5 (Lrp5) or Lrp6 stabilizes β-catenin protein that in turn translocates to the nucleus to bind to T-cell factor (TCF) family of transcription factors to activate gene expression of tissue- and context-specific targets encoding for GS, Cyp1a2, Cyp2e1 and Cyclin-D1. β-Catenin can also be activated in a Wnt-independent manner. Some examples include β-catenin activation by hepatocyte growth factor (HGF) through phosphorylation at tyrosine-654 (Y654) (8), epidermal growth factor (EGF) by phosphorylation at Y654 (9, 10), Flt3 also by phosphorylation at Y654 (11), and protein kinase A (PKA) by phosphorylation at serine-552 (S552) and S675 (12, 13).
In the current study, in order to address to what extent β-catenin signaling in liver is Wnt-signaling dependent, we generated liver-specific Lrp5/6 double knockout or Lrp-LKO mice where upstream Wnt-signaling is disrupted while β-catenin expression is intact, therefore β-catenin can still be activated by pathways other than Wnts. We found that Lrp-LKO mice phenocopied β-catenin-LKO in defective hepatic zonation and delay in LR, which suggests that β-catenin is primarily regulated by Wnt proteins in liver homeostasis and during LR. To address the cellular source of Wnt-proteins in liver, we used Wntless-floxed mice. Wntless (Wls) encodes a multipass transmembrane protein that is specific and necessary for Wnt transport from Golgi to the membrane for secretion (14). Cell-specific Wls deletion has divulged important roles in the development of retina, brain and bone (15–17). We generated Wls conditional KO for liver epithelial cells (Wls-LKO), endothelial cells (Wls-EKO) and macrophages including Kupffer cells (Wls-MKO). We show that Wls-EKO was lethal during development, while heterozygous showed no decrease in Wls protein. Wls-LKO and Wls-MKO mice were viable but lacked any defect in hepatic zonation. Wls-LKO mice exhibit normal initiation of LR. However a temporal defect in LR initiation was evident in Wls-MKO. Thus, we have identified an important cellular circuitry regulating β-catenin activation in liver at baseline and after PH.
EXPERIMENTAL PROCEDURES
Mice and Breeding
All animal experiments and procedures were performed under the strict guidelines of the National Institutes of Health and after approval by the Institutional Animal Use and Care Committees at the University of Pittsburgh and the Van Andel Research Institute. Homozygous Lrp5/6 double-floxed mice were reported recently (18). To conditionally delete Lrp5 and Lrp6 from hepatocytes and cholangiocytes, homozygous Lrp5/6 double-floxed mice (Lrp5flox/floxLrp6flox/flox) were bred to Cre transgenic mice driven by an albumin promoter (Albumin-Cre) (Jackson Laboratories, Bar Harbor, ME) (19). The offspring carrying homozygous Lrp5 floxed alleles, a floxed Lrp6 allele and an albumin-Cre allele (Lrp5flox/floxLrp6flox/wtAlb-Cre+/−) were then bred to homozygous Lrp5/6 double-floxed mice (Lrp5flox/floxLrp6flox/flox). The mice with genotype Lrp5flox/floxLrp6flox/floxAlb-Cre+/− represent liver-specific Lrp5/6 KO or Lrp-LKO mice. Other genotypes Lrp5flox/wt; Lrp6flox/wt; Alb-Cre−/− & Lrp5flox/flox; Lrp6flox/lwt; Alb-Cre−/− & Lrp5flox/wt; Lrp6flox/flox; Alb-Cre−/− & Lrp5flox/flox; Lrp6flox/flox; Alb-Cre−/− were used as controls (Con). No phenotype was observed in Con. Genotyping was performed by polymerase chain reaction (PCR) analysis using genomic DNA isolated from a tail clipping as available in the online supplement.
In order to generate liver-specific Wls KO, homozygous Wls floxed mice (Wlsflox/flox) were bred with Albumin-Cre+/− mice (Jackson Laboratories, Bar Harbor, ME) (19). The offspring carrying floxed Wls allele and Albumin-Cre (Wlsflox/wt; Alb-Cre+/−) were bred to homozygous Wls floxed mice (Wlsflox/flox). Mice with genotype Wlsflox/flox; Alb-Cre+/− represent Wls-LKO mice. Mice with genotypes Wlsflox/flox; Alb-Cre−/− and Wlsflox/wt; Alb-Cre−/− were used as Con.
To generate macrophage-specific Wls KO mice, Wlsflox/flox mice were bred with Lyz2-Cre+/− (also called LyzM-Cre) mice (Jackson Laboratories, Bar Harbor, ME) (20) using similar strategy as described above. Wlsflox/flox; Lyz2-Cre+/− represent Wls-MKO and Wlsflox/flox; Lyz2-Cre−/− and Wlsflox/wt; Lyz2-Cre−/− as Con.
To generate endothelial cell specific Wls knockout mice, Wlsflox/flox mice were bred to Tie2-Cre+/− mice (Jackson Laboratories, Bar Harbor, ME) (21) in the same manner as above to obtain Wlsflox/flox; Tie2-Cre+/− or Wls-EKO mice. Since, no viable pups for Wls-EKO were obtained, mice with genotype Wlsflox/wt; Lyz2-Cre+/− or heterozygous Wls-EKO were used for experiments and mice with phenotypes Wlsflox/flox; Lyz2-Cre−/− and Wlsflox/wt; Lyz2-Cre−/− were used as Con.
Partial Hepatectomy
Twelve-week-old male Con or KO (Lrp-LKO, Wls-LKO, Wls-MKO or heterozygous Wls-EKO) were subjected to partial hepatectomy (PH) (22). Equal numbers of KO and Con mice were killed by cervical dislocation after Isoflurane anesthesia at different time points: 4 hours (n=3), 24 houses (n=3), 40 hours (n≥3), 3 days (n≥3), 4 days (n=3) and 5 days (n=3) after PH. The regenerating livers were harvested and used for protein extraction, and paraffin embedding as described elsewhere (23).
Separation and staining of macrophages
Non-parenchymal cells from mouse liver were isolated by 2-step collagenase perfusion (24). Macrophage positive selection from non-parenchymal cells was performed using QuadroMACS column separation Kit (Miltenyi Biotech, Cambridge, MA). Anti-mouse F4/80 antibody (Biolegend, San Diego, CA) and specific microbeads were used according to the manufacture’s instruction. After the column separation of macrophages, cytospin was performed. Cells were centrifuged at 500 rpm for 5 minutes on glass slides followed by fixation with 4% paraformaldehyde for 10 minutes. Rat anti-mouse F4/80 antibody (AbD Serotec, Raleigh, NC) was used for immunofluorescent staining as described elsewhere (25).
Additional methods in online supplement
RESULTS
Generation and characterization of conditional Lrp5/6 null mice
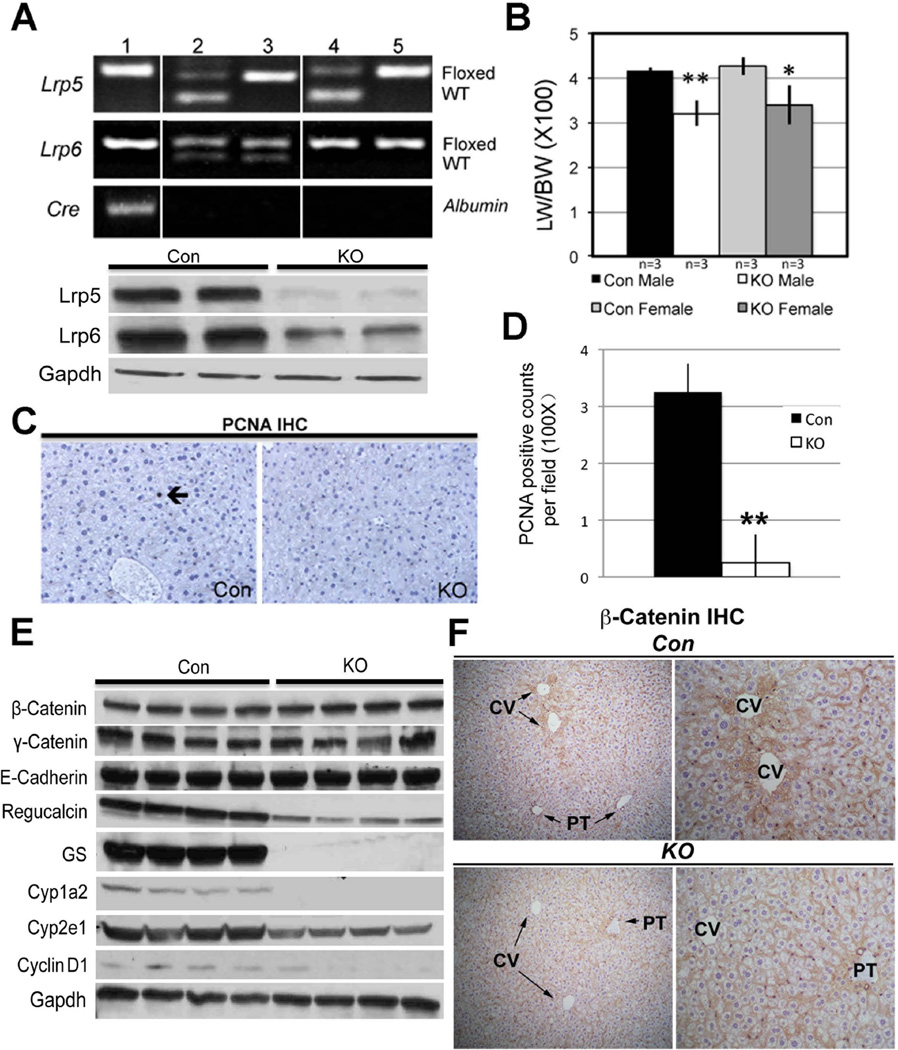
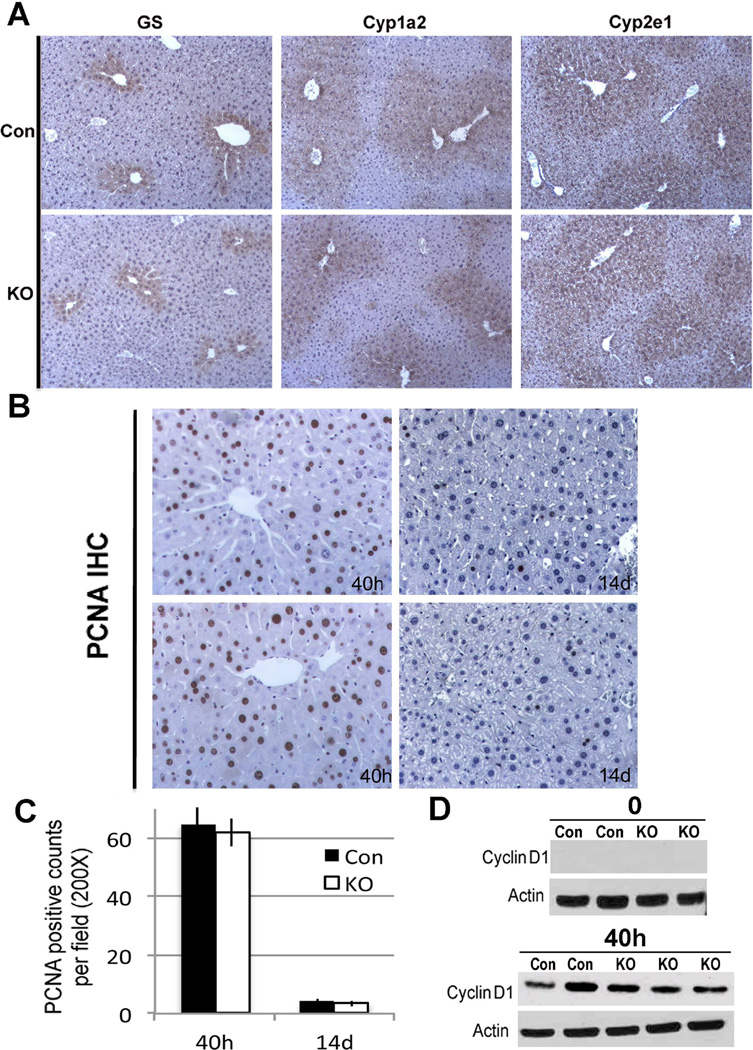
After strategic breeding, liver-specific Lrp5/6 null mice (Lrp-LKO) were generated. Genotyping was performed by PCR (Figure 1A), using primer P1 and P3 described previously (18). Livers from Lrp-LKO exhibited a significant decrease in Lrp5 and Lrp6 protein compared to control mice (Con) (Figure 1A). Eight-month-old Lrp-LKO and Con mice had comparable and normal levels of serum AST, ALT and albumin (data not shown). While total bilirubin was within normal range, average in controls was 0.26 mg/dl (n=5) as compared to 0.5 mg/dl (n=5) in Lrp-LKO (p=0.009). Lrp-LKO also displayed smaller livers and LW/BW ratio was significantly lower in Lrp-LKO compared to Con (Figure 1B). To address the discrepancy in size, we assessed hepatocytes in S-phase by PCNA immunohistochemistry (IHC). Lrp-LKO livers had significantly less PCNA positive cells at baseline (Figure 1C, 1D). Concomitantly, lower Cyclin-D1 expression was also evident in KO by western blots (WB) (Figure 1E). Intriguingly, all the above observations were similar to β-catenin-LKO mice (6).
Figure 1. Successful deletion of Lrp5 and Lrp6 in liver leads to alterations in downstream β-catenin signaling. (*p<0.05, **p<0.01).
A. Representative PCR (upper panel) shows genotype of mice for identifying Lrp-LKO with genotype Lrp5flox/flox; Lrp6flox/flox; Cre+/− (lane 1). Mice with other genotypes (lane 2–5) were used as Con. Representative WB (lower panel) shows a dramatic decrease in Lrp5 and Lrp6 in the tissue lysates from the Lrp-LKO livers.
B. Decreased LW/BW ratio in 8-month old Lrp-LKO. This difference was around 20% in both males and females and significant.
C. IHC for PCNA shows an occasional positive hepatocyte (arrow) in a representative section from Con liver at baseline, while none were detected in Lrp-LKO.
D. Quantification of PCNA staining shows significantly fewer positive cells in Lrp-LKO.
E. Representative WB shows no change in total levels of β-catenin, γ-catenin and E-cadherin in Lrp-LKO livers as compared to Con. However several downstream targets of β-catenin were downregulated in Lrp-LKO livers. Prolonged exposure of WB film enabled us to observe baseline Cyclin-D1 in Con, which was notably reduced in Lrp-LKO livers.
F. IHC for β-catenin shows predominantly membranous localization in both Lrp-LKO and Con livers. However, in Con, cytosolic localization of β-catenin was seen in hepatocytes around central vein (CV) and not portal triads (PT). Lrp-LKO show absence of cytosolic β-catenin around CV with a few hepatocytes around PT showing some cytoplasmic localization. (left panel Con and KO−100×; right panel Con and KO−200×)
β-Catenin at adherens junctions in the Lrp-LKO livers at baseline
Next, we assessed β-catenin levels and levels of other key components at adherens junctions in the Lrp-LKO livers. WB shows Lrp-LKO and Con to have comparable levels of β-catenin, γ-catenin and E-cadherin (Figure 1E).
Disruption of metabolic zonation in conditional Lrp5/6 null mice
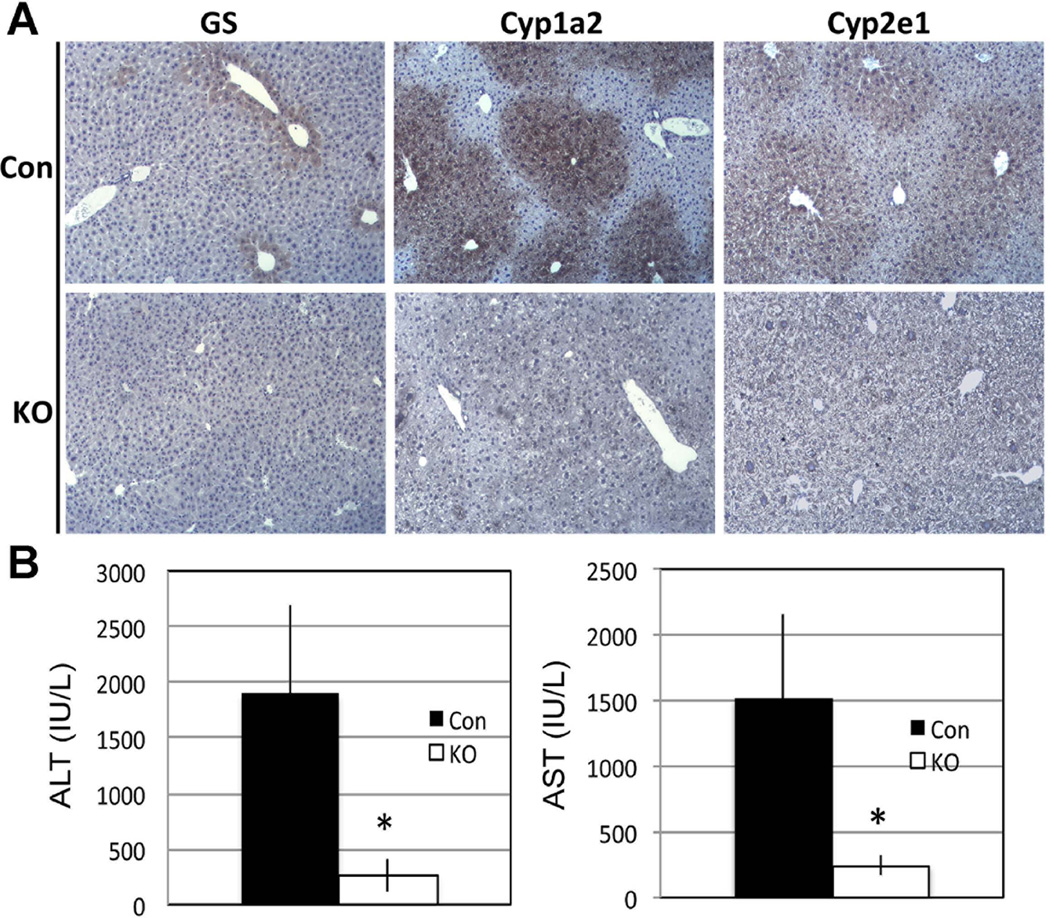
Next, we assessed β-catenin localization by IHC in Con and Lrp-LKO livers. Con livers show predominantly membranous β-catenin except in pericentral hepatocytes where there was enhanced cytosolic labeling as well (Figure 1F). However in Lrp-LKO, cytosolic β-catenin localization in pericentral hepatocytes was notably lacking with occasional periportal hepatocyte showing some cytoplasmic staining (Figure 1F). Lrp-LKO livers also showed notably low protein expression of multiple downstream targets of β-catenin, such as Regucalcin, GS, Cyp1a2 and Cyp2e1 (Figure 1E). IHC also showed loss of pericentral expression of GS, Cyp2e1 and Cyp1a2 in Lrp-LKO livers (Fig. 2A).
Figure 2. Loss of Lrp5/6 in liver compromises pericentral zonation (*p<0.05).
A. IHC shows normal pericentral expression of GS, Cyp1a2 and Cyp2e1 in Con livers, while absence of these proteins is observed in littermate Lrp-LKO livers.
B. When injected with lethal dose of APAP (600 mg/kg body weight), Lrp-LKO showed significantly lower ALT and AST levels than Con after 24 hours.
The functional loss of Cyp1a2 and Cyp2e1 was assessed by acetaminophen (APAP) toxicity study. Cyp1a2 and Cyp2e1 metabolize APAP to a toxic metabolite, N-acetyl-p-benzo-quinone imine (NAPQI) to cause hepatocyte necrosis through glutathione depletion (26). β-Catenin-LKO mice are protected from APAP overdose due to absent Cyp2e1 and Cyp1a2 (5, 27). Lrp-LKO also showed significantly lower serum AST and ALT as compared to Con after APAP (600mg/kg) administration (Figure 2B). Therefore, despite normal β-catenin expression in Lrp-LKO, ablation of Lrp5/6 disrupts Wnt signaling to impair β-catenin activity and zonation.
Retarded liver regeneration in Lrp-LKO mice after partial hepatectomy
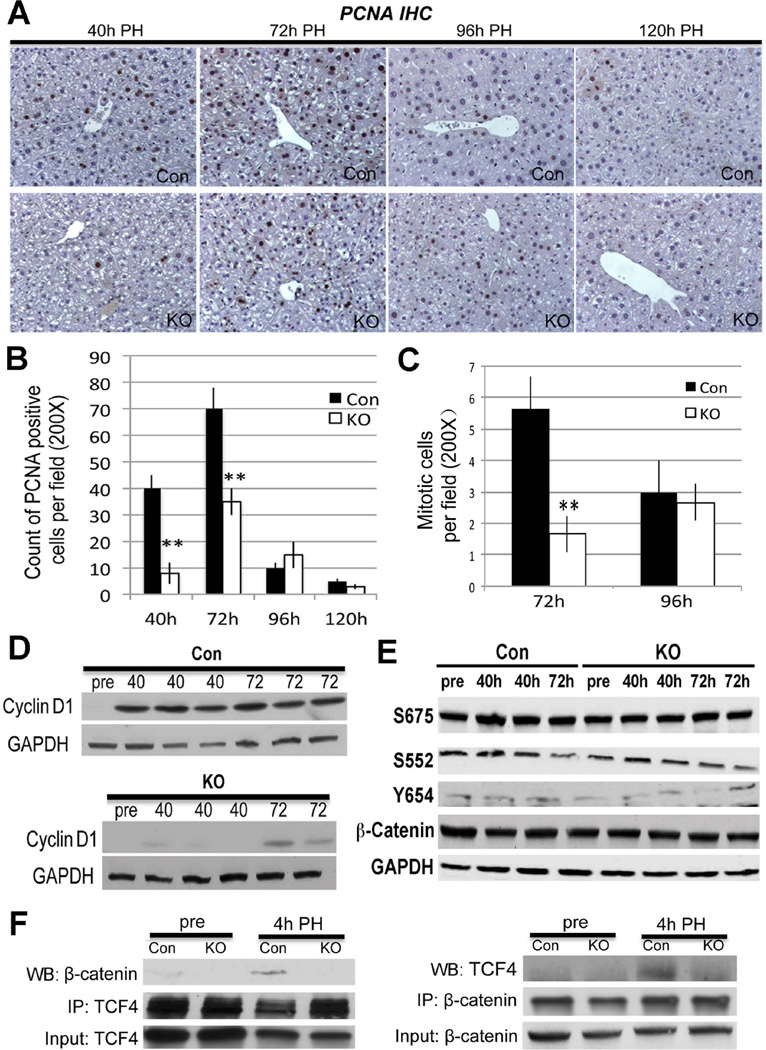
Next, Lrp-LKO and Con were subjected to PH. As expected, Con showed abundant hepatocytes in S-phase at 40 and 72 hours after PH with decline at later time-points (Figure 3A, 3B). Intriguingly, Lrp-LKO livers exhibited significantly fewer PCNA-positive hepatocytes at both 40 and 72 hours, although more PCNA-positive hepatocytes were observed at 72 than 40 hours (Figure 3A, 3B). In addition, numbers of mitotic figures as assessed by analysis of H&E images from Lrp-LKO and Con showed a notably lower mitosis in hepatocytes in the former at 72 hours (Figure 3C and Online Supplement Figure 1A). β-Catenin target Cyclin-D1 that regulates G1-S phase transition during LR (28) was also lower in Lrp-LKO at 40 hours with progressive increase at 72 hours although it was still lower than Con (Figure 3D).
Figure 3. Abolishing Wnt/β-catenin signaling through Lrp5/6 ablation in liver impairs LR after PH.
A. IHC for PCNA identifies several hepatocytes in S-phase at 40 and 72 hours after PH, while only a few trailing hepatocytes were PCNA-positive at 96 and 120 hours. In contrast, Lrp-LKO showed dramatically fewer PCNA-positive hepatocytes at 40 hours with an increase at 72 hours. Like Con, only a few PCNA-positive hepatocytes were detected at later times in Lrp-LKO. (200×)
B. PCNA quantification shows a gradual increase in positive hepatocytes in Lrp-LKO from 40 to 72 hours although these are lower than Con at both times (**p<0.01).
C. Quantification of mitotic figures shows a significantly lower numbers in Lrp-LKO as compared at Con at 72 hours after PH. (**p<0.01)
D. WB shows low protein expression of Cyclin-D1 after PH in Lrp-LKO compared to Con at both 40 and 72 hours although its levels increase in Lrp-LKO at 72 hours.
E. Representative WB shows comparable levels of S675-β-catenin, S552-β-catenin, Y654-β-catenin and total β-catenin in Con and Lrp-LKO livers after PH.
F. Representative WB shows β-catenin and TCF4 association at 4 hours after PH in Con and not Lrp-LKO livers by immunoprecipitation studies. Immunoprecipitation studies were performed by pull down of either β-catenin or TCF4. Respective input controls are included in analysis as well.
We wanted to address if there was compensatory activation of β-catenin by alternate signals such as HGF, EGF or PKA, in the absence of Wnt. However, no differences in levels of Y654-, S552- or S675-β-catenin were evident between Lrp-LKO and Con at 40 or 72 hours after PH (Figure 3E).
Since β-catenin binding to TCF4 precedes Cyclin-D1 expression after PH (6, 24), we assessed their association by immunoprecipitation at 4 hours after PH. TCF4-β-catenin complex was observed in Con but absent in Lrp-LKO (Figure 3F).
Taken together, these data suggest that Lrp-LKO phenocopy β-catenin-LKO after PH, thereby indicating that only Wnt signaling is responsible for β-catenin activation during LR after PH.
Generation and baseline characterization of liver-specific Wls KO
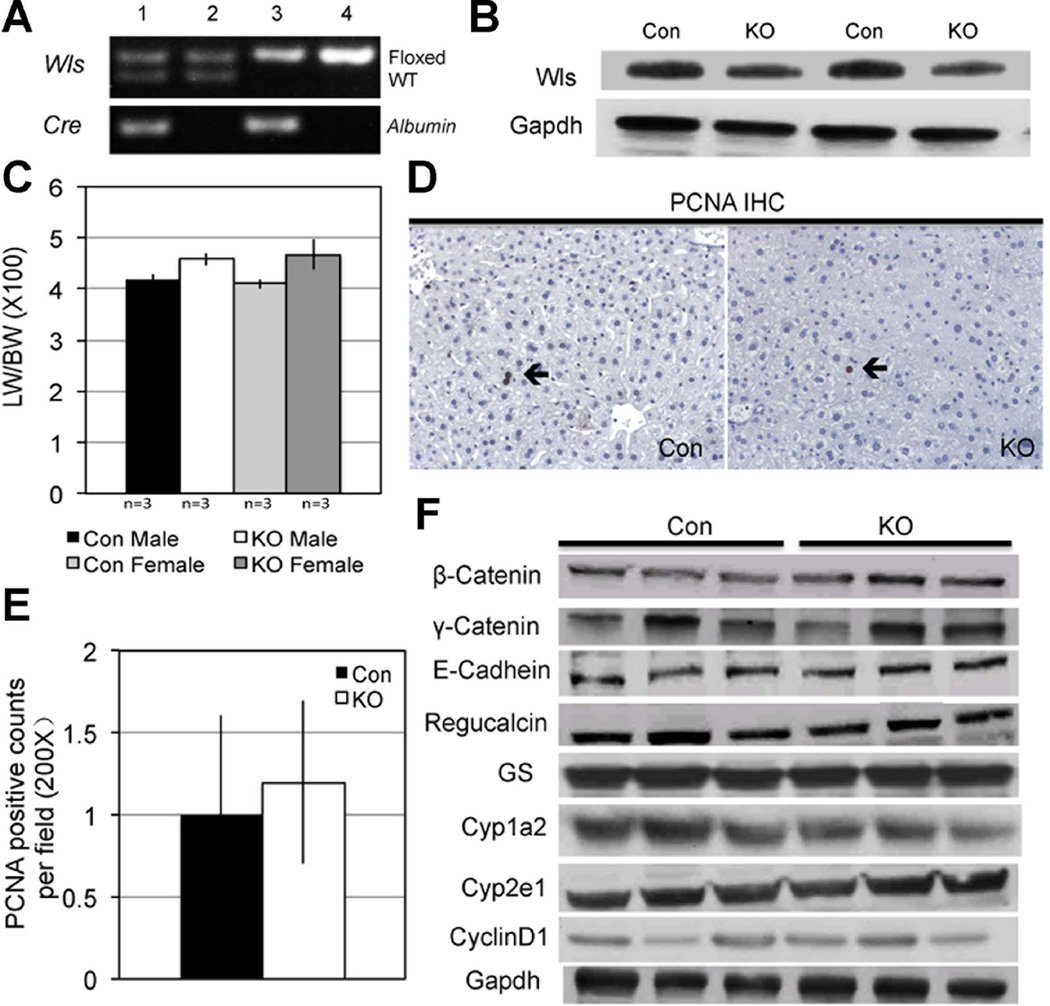
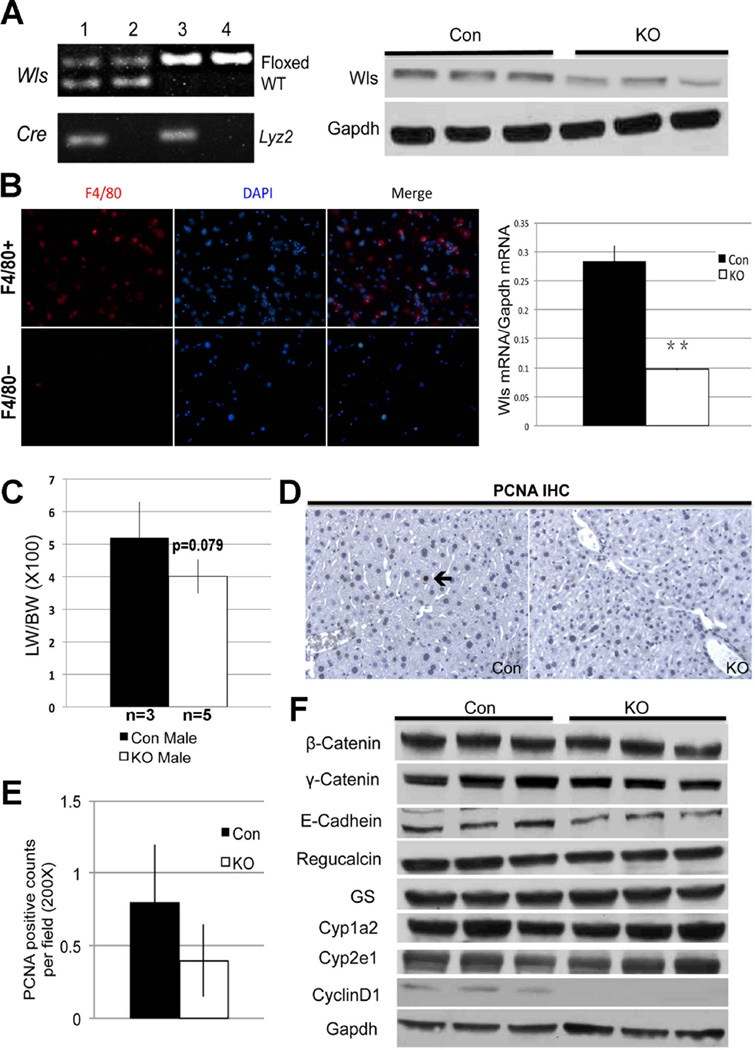
To address the cell source of Wnt proteins directing β-catenin activity for zonation and during LR, we first generated hepatocyte and cholangiocyte (or liver-specific) Wls KO (Wls-LKO) by interbreeding Albumin-Cre and Wls-floxed mice. PCR confirmed the concomitant presence of floxed allele and cre-recombinase in Wls-LKO (Figure 4A), using primer P2 and P4 described previously (15). WB from Wls-LKO liver lysates show less Wls relative to Con (Figure 4B). However, appreciable Wls persisted in Wls-LKO suggesting that other non-parenchymal cells also express Wls, which would not be affected by Albumin-cre. Indeed, cell fractionation into parenchymal and non-parenchymal cells by percoll gradient after collagenase perfusion showed notably higher Wls expression in the non-parenchymal cell compartment (data not shown).
Figure 4. Successful deletion of Wls in Wls-LKO mice.
A. Representative PCR shows genotype of mice for identifying the Wlsflox/flox; Albumin-Cre+/− (Wls-LKO) (lane 3). Mice with genotype Wlsflox/wt; Albumin-Cre−/− & Wlsflox/flox;Albumin-Cre−/− (lanes 2 & 4) were used as Con.
B. WB shows a modest decrease in Wls in liver of Wls-LKO compared to Con.
C. Higher LW/BW ratio in 8-month old Wls-LKO as compared to Con although difference was not significant.
D. IHC shows occasional PCNA-positive hepatocyte in Con and Wls-LKO liver (200×).
E. Quantification of PCNA staining (200×) shows comparable number of positive hepatocytes between Con and Wls-LKO.
F. Representative WB shows comparable expression of β-catenin, relevant junctional components and β-catenin downstream, targets in Wls-LKO and Con livers. Prolonged exposure of WB film enabled us to observe baseline Cyclin-D1 in Wls-LKO and Con which was comparable in the two groups.
Eight-month-old Wls-LKO mice had comparable and normal levels of serum AST, ALT, bilirubin, and albumin levels to Con (data not shown). Wls-LKO showed marginally bigger livers than Con (Figure 4C), although the difference was not statistically significant. Baseline proliferation examined by IHC for PCNA did not reveal any differences (Figure 4D, 4E).
Normal adherens junctions and hepatic zonation in Wls-LKO null mice
Next, we investigated changes in β-catenin, its downstream signaling and junctional proteins in Wls-LKO mice. β-Catenin, γ-catenin and E-cadherin were unchanged in Wls-LKO (Figure 4F). The expression of the downstream targets of β-catenin signaling was comparable between Wls-LKO and Con (Figure 4F). IHC for pericentral expression of β-catenin targets showed no differences in staining for GS, Cyp1a2 and Cyp2e1 between Wls-LKO and Con (Figure 5A). Therefore, blocking Wnt secretion from hepatocytes or cholangiocytes does not affect regulation of zonation by β-catenin signaling in hepatocytes.
Figure 5. Ablation of Wls in liver epithelial cells has no effect on hepatic zonation or initiation of LR.
A. IHC for GS, Cyp1a2 and Cyp2e1 in Con and Wls-LKO shows normal pericentral localization in both groups. (100×)
B. PCNA IHC shows comparable numbers of PCNA-positive hepatocytes in Con and Wls-LKO livers at 40 hours after PH (200×).
C. Quantification of PCNA staining confirms similar numbers of PCNA-positive hepatocytes in Con and Wls-LKO at 40 hours after PH.
D. Representative WB shows comparable Cyclin-D1 expression at 40 hours after PH in both Con and Wls-LKO livers.
Normal initiation of liver regeneration in Wls-LKO null mice
The role of hepatocyte- and cholangiocyte-derived Wnt proteins in LR was examined next by subjecting the Wls-LKO and Con to PH. Comparably higher numbers of PCNA-positive hepatocytes were observed in Wls-LKO and Con mice at 40 hours post-PH (Figure 5B). Quantification of PCNA staining showed no significant differences (Figure 5C). Likewise, Wls-LKO had comparable Cyclin-D1 levels to Con at 40 hours (Figure 5D). Analysis at 14 days after PH when liver is known to have regenerated to its pre-hepatectomy mass also revealed no difference in PCNA-positivity between the Wls-LKO and Con group (Figure 5B and 5C). Therefore, lack of any differences in regeneration kinetics at early and late times after PH indicate that β-catenin activation during LR is not dependent on hepatocyte- or cholangiocyte-derived Wnts.
Generation of endothelial cell-specific Wls null mice
Deletion of Wls using Tie2-cre led to no viable pups suggesting embryonic lethality. Heterozygous mice that lacked an allele of Wls in endothelial cells showed no decrease in Wls protein and these livers did not show any decrease in pericentral targets (data not shown). When subjected to PH, no defect in LR was observed in heterozygous Wls-EKO (data not shown).
Generation and baseline characterization of macrophage-specific Wls null mice
We next bred the Wls-floxed mice to macrophage-specific Lyz2-Cre mice (29). PCR was used to identify the Wls-MKO and Con (Figure 6A). Decreased level of Wls protein was evident in WB from Wls-MKO livers (Figure 6A). To verify successful loss of Wls, Kupffer cells were isolated by QuadroMACS column separation as discussed in methods and stained for marker F4/80 to demonstrate a notable enrichment (Figure 6B). Quantitative RT-PCR performed on enriched Kupffer cell-fraction showed a significant decrease in Wls expression in Wls-MKO as compared to Con (Figure 6B).
Figure 6. Impact of deletion of Wls in Kupffer cells on hepatic homeostasis.
A. Representative PCR (left) showing genotype of Wls-MKO (Wlsflox/flox; Lyz2-Cre+/−) (lane 3) and Con with genotype Wlsflox/wt; Lyz2-Cre−/− & Wlsflox/flox;Lyz2-Cre−/− (lanes 2 & 4). Representative WB shows a notable decrease in Wls in Wls-MKO liver lysate.
B. Enrichment for Kupffer cells after collagenase perfusion and column separation of non-parenchymal cells is verified by immunocytochemistry on cytospin for F4/80 (red) showing positive staining in the F4/80-positive cell fraction and not in F4/80-negative fraction (left). Wls mRNA expression in F4/80+ cells isolated from Con and Wls-MKO, shows significantly lower expression in the latter (**p<0.001).
C. Bar graph depicts marginally lower LW/BW ratio in Wls-MKO, although difference was not statistically significant.
D. IHC (200×) shows an occasional PCNA-positive hepatocyte in Con at baseline (arrow), while no positive cell is observed in a representative view in Wls-MKO.
E. Quantification of PCNA staining showing less positive cells in Wls-Mac KO than Con at baseline, although differences were insignificant.
F. WB shows no alteration in proteins levels of β-catenin, junctional proteins and pericentral targets. However Cyclin-D1 was notably lower in Wls-MKO livers.
Eight-month-old Wls-MKO and Con mice had comparable and normal serum levels of AST, ALT, bilirubin and albumin (data not shown). Wls-MKO mice (n=5) had marginally smaller livers than Con (n=3); however the difference did not reach statistical significance (p=0.079) (Figure 6C). IHC for PCNA identified fewer positive cells in Wls-MKO, although differences were not statistically significant (Figure 6D, 6E). Intriguingly, a notable decrease in baseline Cyclin-D1 protein expression was evident in Wls-MKO (Figure 6F). Hence, we conclude that macrophages may secrete Wnts and contribute to baseline hepatocyte turnover to govern liver size.
Normal adherens junctions and hepatic zonation in Wls-MKO mice
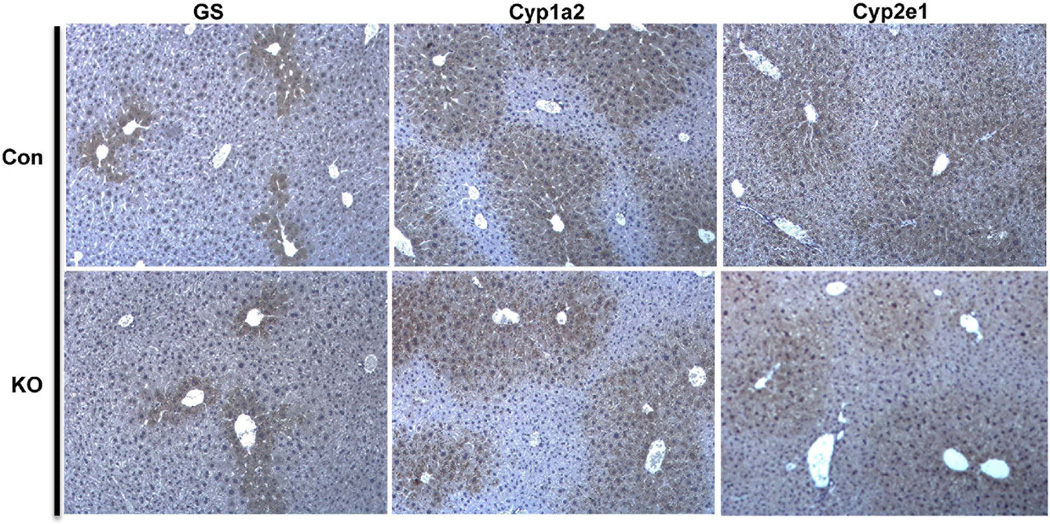
Next, the effect of abolishing Wnt secretion from macrophages on β-catenin levels and other related junctional proteins was assessed. WB showed no notable changes in the levels of β-catenin, γ-catenin or E-cadherin in Wls-MKO when compared to Con livers (Figure 6F). WB also shows comparable levels of Regucalcin, GS, Cyp1a2 & Cyp2e1 in Wls-MKO and Con livers (Figure 6F). More importantly, comparable GS, Cyp1a2 and Cyp2e1 localization confined to centrilobular area was evident in Wls-MKO and Con livers (Figure 7). Thus, blocking Wnt secretion from macrophages does not have any effect on β-catenin at adherens junctions or in metabolic zonation.
Figure 7. No defect in pericentral zonation in Wls-MKO.
IHC shows normal pericentral localization of GS, Cyp1a2 and Cyp2e1 in Con and Wls-Mac KO livers.
Suboptimal liver regeneration in Wls-MKO mice
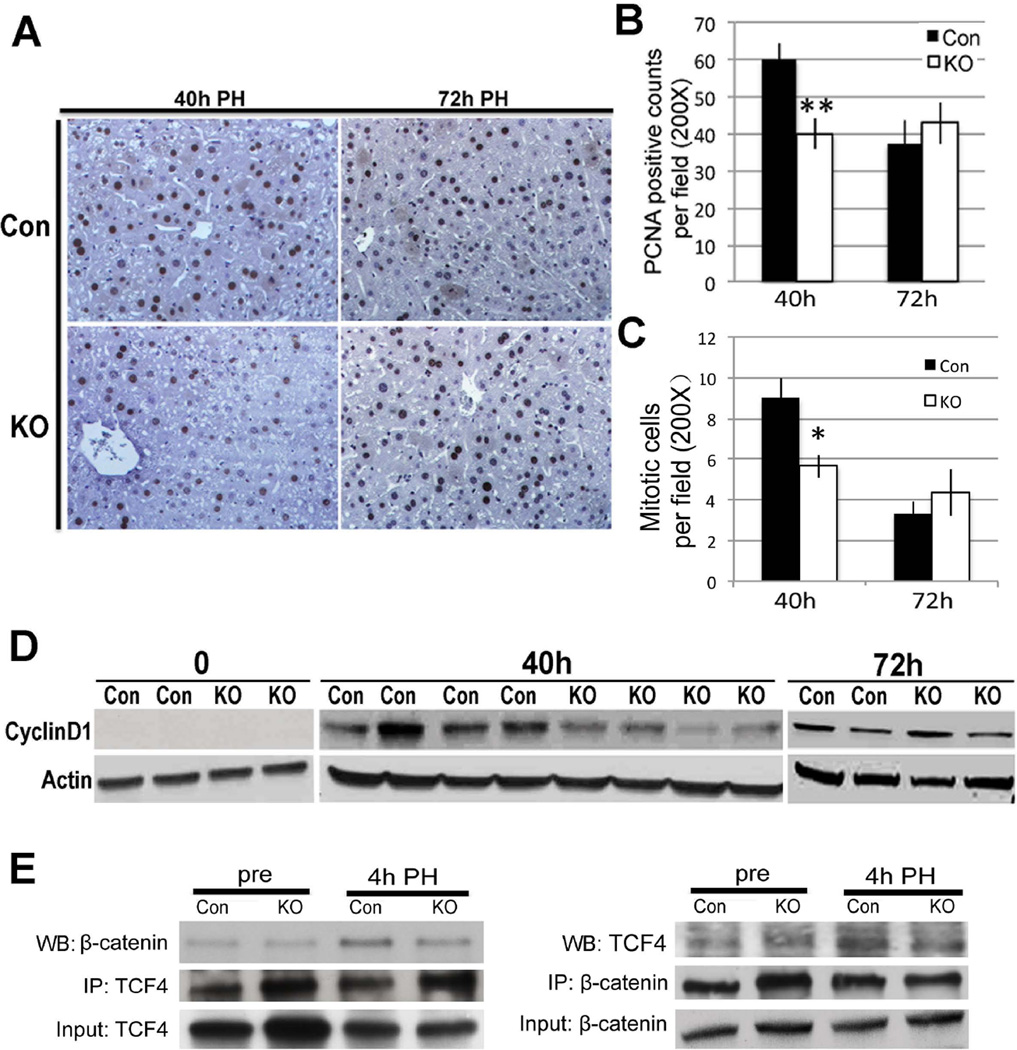
To address role of macrophage-derived Wnts in LR, Wls-MKO and Con were subjected to PH. At 40 hours after PH, a notable decrease in PCNA-positive hepatocytes was observed in Wls-MKO, however at 72 hours no difference was seen (Figure 8A). Upon quantification, this deficit at 40 hours was found to be around 33% and significant (Figure 8B). In addition, numbers of mitotic figures as assessed by analysis of H&E images from Wls-MKO and Con showed significantly lower mitosis in hepatocytes in the former at 40 hours (Figure 8C and Online Supplement Figure 1B). WB shows a concomitant decrease in Cyclin-D1 at 40 hours in Wls-Mac KO (Figure 8D). Coprecipitation studies showed a notable decrease in β-catenin-TCF4 complex in Wls-MKO livers at 4 hours after PH (Figure 8E). This indicates that β-catenin activation in hepatocytes after PH is at least in part regulated by Wnts derived from Kupffer cells.
Figure 8. Dampened LR in Wls-MKO after PH.
A. IHC (200×) showing many PCNA-positive hepatocytes in Con livers at 40 hours and 72 hours; however several PCNA-negative hepatocytes were observed in Wls-MKO at 40 hours.
B. Quantification of PCNA staining showing a 33% and significant decease in the number of hepatocytes in S-phase in Wls-MKO at 40 hours (**p<0.01).
C. Quantification of mitotic figures shows a significantly lower numbers in Lrp-LKO as compared at Con at 72 hours after PH. (*p<0.05)
D. Representative WB shows lower Cyclin-D1 levels in Wls-MKO at 40 hours after PH.
E. A representative immunoprecipitation study shows decreased TCF4-β-catenin complex formation at 4 hours after PH in Wls-Mac KO when compared to Con. Immunoprecipitation studies were performed by pull down of either β-catenin or TCF4. Respective input controls are included in analysis as well.
DISCUSSION
β-Catenin, the critical downstream effector of Wnt signaling, acts as a co-activator for TCF family of transcription factors to regulate expression of several target genes in a tissue specific manner. Deletion of β-catenin in hepatocytes has yielded two major phenotypes in the liver (4–6). The first observation at baseline is a defect in metabolic zonation as β-Catenin-LKO mice lack several downstream targets in pericentral hepatocytes such as genes encoding for GS (ammonia metabolism), Cyp2e1, Cyp1a2 (xenobiotic metabolism) and others involved in glycolytic pathway and Tricarboxylic acid (TCA) cycle (30). The second phenotype in β-catenin-LKO mice was a defect in LR after PH. The role of β-catenin signaling in LR is now well-established in rodents, zebrafish and patients (2, 31). Activation of β-catenin is relatively early with its nuclear translocation evident in minutes to hours after PH, where it complexes with TCF4 to regulate the expression of Ccnd1. Cyclin-D1 is critical for driving G1 to S phase cell cycle progression and marks the point of no return for cell proliferation (32). In fact absence of β-catenin in hepatocytes in β-Catenin-LKO led to a significant lag in the initiation of LR with hepatocyte S-phase peaking at 72 hours instead of 40 hours due to decreased Ccnd1 (4, 6).
Endogenous or exogenous activation of β-catenin has been shown to improve LR in animal models as well as in patients (24, 27, 33). However, for translational exploitation of β-catenin signaling in regenerative medicine, it will be critical to understand the cellular and molecular circuitry upstream of β-catenin activation in hepatocytes at baseline and during LR. This is of special relevance since β-catenin activation has been also shown to occur downstream of non-Wnt mechanisms such as those mediated by HGF, EGF, Flt3 and PKA, which have been independently shown to be playing a significant role in LR after PH (34).
In the current study using genetic mouse models we address the key regulators of β-catenin in hepatic zonation and LR. We used genetic KO of Lrp5 and Lrp6 to disrupt Wnt signaling in hepatocytes and cholangiocytes using Albumin-Cre, which was also used by us to delete β-catenin in the same two cell-types previously (6, 35). Deleting both Lrp5 and Lrp6 has been shown to prevent any compensation and hence assures complete abrogation of Wnt signaling (18). This breach in Wnt signaling in hepatocytes prevented pericentral β-catenin activation and led to loss of GS, Cyp1a2 and Cyp2e1, which phenocopied β-catenin-LKO. This finding demonstrates that the pericentral zonation function of β-catenin is fully regulated by Wnt signaling. A more surprising result came from the analysis of LR in this model. Despite β-catenin activation by many non-Wnt dependent mechanisms and despite the physiological activation of such pathways as HGF, EGF and PKA after PH, there was a considerable deficit in LR in the Lrp-LKO similar to β-catenin-LKO mice as seen by decreased PCNA at 40 and 72 hours and reduced mitosis at 72 hours (4, 36). Intriguingly, while β-catenin-LKO showed comparable number of hepatocytes in S-phase to Con at 72 hours, the Lrp-LKO continued to show fewer hepatocytes in S-phase at this time as compared to their littermate controls. However the numbers of hepatocytes in S-phase were notably greater at 72 hours than the 40-hour time-point, suggesting redundant mechanisms that compensate, which was what was observed in β-catenin-LKO as well. An increase in Cyclin-D1 at 72 hours in regenerating Lrp-LKO livers over baseline and 40 hours suggests sufficient LR to restore the smaller baseline hepatic mass in Lrp-LKO. No alternate mechanism of β-catenin activation in regenerating Lrp-LKO livers was observed at any time. Thus Wnt signaling strictly regulates β-catenin during the process of LR.
To determine cellular source of Wnts at baseline and after PH is challenging as several hepatic cells express multiple Wnts (37). An exciting development to address the role of global Wnt signaling has been to interfere with either their biological activity or prevent their secretion from a cell. Deletion of the gene encoding for porcupine, which is responsible for glycosylation and acylation of Wnt proteins, has been used to disrupt Wnt function (38). Another relevant protein Wntless has been shown to be indispensable for Wnt secretion (14). In fact, conditional deletion of Wls using Wnt1-cre phenocopies Wnt1-null abnormalities in brain (15, 39). Utilizing Wls-floxed mice, we blocked Wnt secretion from hepatocytes and cholangiocytes, Kupffer cells, and endothelial cells. Our results show no alteration in either pericentral gene expression, or in the initiation of LR when Wnt-secretion was ablated from hepatocytes and cholangiocytes. Tie2-Cre mediated Wls deletion led to embryonic lethality, which was not surprising because of the many important roles of Wnt/β-catenin signaling in vasculogenesis and angiogenesis (40, 41). While embryonic lethality precluded us from addressing the role of hepatic sinusoidal endothelial cells and of endothelial cells lining the hepatic vessel walls, viable heterozygous Wls-EKO mutants did not show any notable decrease in Wls. Simultaneously, no defects in hepatic zonation or LR were observed. Current studies are ongoing to generate inducible-conditional KO to address the endothelial-hepatocyte paracrine interactions in the liver. It should be noted that recent studies have divulged a role of platelet-sinusoidal endothelial cell-hepatocyte circuitry to activate β-catenin by Wnt2b during LR (42).
A more consequential observation came from abrogating Wnt secretion from Kupffer cells. While no defect in metabolic zonation was observed, a 33% decrease in S-phase hepatocytes and hepatocyte mitosis was evident in Wls-MKO at 40 hours after PH. This coincided with a notable decrease in β-catenin-TCF4 complex and Cyclin-D1 expression at 40 hours. These observations suggest that Kupffer cells are an important source of Wnt proteins that activate hepatocyte β-catenin in a paracrine manner. Indeed the role of macrophages in initiation of LR is unquestionable (43).
Thus, our current study demonstrates the regulation of β-catenin in two fundamental processes inherent to the liver: hepatic zonation and regeneration. In both cases, β-catenin is under the control of Wnt signaling only. Our studies rule out the role of hepatocytes, cholangiocytes and Kupffer cells as the source of Wnt proteins that regulate basal pericentral β-catenin activation in liver. On the other hand, it became apparent that Wnt secretion from non-parenchymal cell compartment especially Kupffer cells and endothelial cells (42), is required for timely β-catenin activation in hepatocytes to initiate LR after PH. Clearly, the significance of non-parenchymal cell contribution to LR is well accepted (44). We hope to determine the role of hepatic stellate cells and further explore the role of sinusoidal and perivenous endothelial cells in β-catenin activation in pericentral hepatocytes in regulating hepatic zonation and during LR after PH.
Supplementary Material
ACKNOWLEDGEMENTS
We also thank Ammar Saladhar and Angie Lake for technical assistance.
FUNDING SUPPORT: This study was funded by NIH grants 1R01DK62277, 1R01DK100287 and Endowed Chair for Experimental Pathology to SPSM. This study was also in part funded by 5R01AR053293 and 5R21RR024887 to BOW.
Abbreviations
- CYP
Cytochrome P450 enzymes
- Evi
Evenness Interrupted
- Wls
Wntless
- LRP
LDL-related protein
- LR
liver regeneration
- PH
partial hepatectomy
- HGF
hepatocyte growth factor
- EGF
epidermal growth factor
- PKA
protein kinase A
- KO
knockout
- β-Catenin-LKO
liver-specific β-catenin knockout
- Lrp-LKO
liver-specific LRP5/6 double KO
- Wls-LKO
liver-specific Wls KO
- Wls-MKO
Macrophage-specific Wls KO
- Wls-EKO
endothelial cell-specific Wls KO
- Con
littermate controls
- LW/BW
liver weight to body weight ratio
- PCR
polymerase chain reaction
- ALT
serum alanine aminotransferases
- AST
aspartate aminotransferase
- TCF
T cell factor
- GS
Glutamine Sythetase
- APAP
Acetaminophen
- PCNA
proliferating cell nuclear antigen
- IHC
immunohistochemistry
- WB
western blot
REFERENCES
- 1.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43:1021–1029. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 5.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 6.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 8.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer research. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 9.Shibata T, Ochiai A, Kanai Y, Akimoto S, Gotoh M, Yasui N, Machinami R, et al. Dominant negative inhibition of the association between beta-catenin and c-erbB-2 by N-terminally deleted beta-catenin suppresses the invasion and metastasis of cancer cells. Oncogene. 1996;13:883–889. [PubMed] [Google Scholar]
- 10.van Veelen W, Le NH, Helvensteijn W, Blonden L, Theeuwes M, Bakker ER, Franken PF, et al. beta-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut. 2011;60:1204–1212. doi: 10.1136/gut.2010.233460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajiguchi T, Katsumi A, Tanizaki R, Kiyoi H, Naoe T. Y654 of beta-catenin is essential for FLT3/ITD-related tyrosine phosphorylation and nuclear localization of beta-catenin. Eur J Haematol. 2012;88:314–320. doi: 10.1111/j.1600-0609.2011.01738.x. [DOI] [PubMed] [Google Scholar]
- 12.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Molecular and cellular biology. 2005;25:9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. The Journal of biological chemistry. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 14.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefater JA, 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109:E2197–E2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, Baker JJ, Zylstra-Diegel CR, Williams BO. Lrp5 and Lrp6 play compensatory roles in mouse intestinal development. Journal of cellular biochemistry. 2012;113:31–38. doi: 10.1002/jcb.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 20.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 21.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 22.Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Molecular and cellular biology. 1999;19:8570–8580. doi: 10.1128/mcb.19.12.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wickline ED, Awuah PK, Behari J, Ross M, Stolz DB, Monga SP. Hepatocyte gamma-catenin compensates for conditionally deleted beta-catenin at adherens junctions. J Hepatol. 2011;55:1256–1262. doi: 10.1016/j.jhep.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Jr, Dar MJ, Khillan J, Dai C, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007;9:951–959. doi: 10.1593/neo.07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raucy JL, Lasker JM, Lieber CS, Black M. Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Archives of biochemistry and biophysics. 1989;271:270–283. doi: 10.1016/0003-9861(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 27.Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. The American journal of pathology. 2009;175:1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albrecht JH, Rieland BM, Nelsen CJ, Ahonen CL. Regulation of G(1) cyclin-dependent kinases in the liver: role of nuclear localization and p27 sequestration. Am J Physiol. 1999;277:G1207–G1216. doi: 10.1152/ajpgi.1999.277.6.G1207. [DOI] [PubMed] [Google Scholar]
- 29.Nikolic T, Movita D, Lambers ME, de Almeida CR, Biesta P, Kreefft K, de Bruijn MJ, et al. The DNA-binding factor Ctcf critically controls gene expression in macrophages. Cell Mol Immunol. 2013 doi: 10.1038/cmi.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torre C, Perret C, Colnot S. Transcription dynamics in a physiological process: beta-catenin signaling directs liver metabolic zonation. Int J Biochem Cell Biol. 2011;43:271–278. doi: 10.1016/j.biocel.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- 32.Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1999;10:397–404. [PubMed] [Google Scholar]
- 33.Fanti M, Singh S, Ledda-Columbano GM, Columbano A, Monga SP. Triiodothyronine induces hepatocyte proliferation by protein kinase A-dependent -catenin activation in rodents. Hepatology. 2013 doi: 10.1002/hep.26775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3:485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- 35.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 36.Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, Monga DK, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng G, Awan F, Otruba W, Muller P, Apte U, Tan X, Gandhi C, et al. Wnt'er in liver: expression of Wnt and frizzled genes in mouse. Hepatology. 2007;45:195–204. doi: 10.1002/hep.21473. [DOI] [PubMed] [Google Scholar]
- 38.Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci U S A. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 40.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107:943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 42.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Meijer S, van Rooijen N, van Lambalgen AA, et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 44.Nejak-Bowen KN, Orr AV, Bowen WC, Jr, Michalopoulos GK. Gliotoxin-induced changes in rat liver regeneration after partial hepatectomy. Liver Int. 2013;33:1044–1055. doi: 10.1111/liv.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.