Fig. 8.
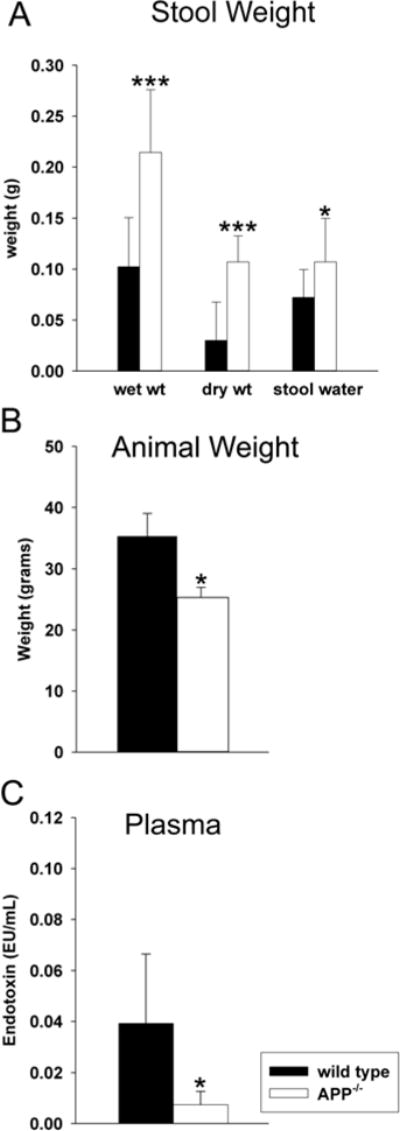
APP−/− mice demonstrated intestinal motility and absorption differences compared to wild type mice. To compare overall intestinal absorption and motility, (A) water and stool production, (B) animal weight and (C) uptake of LPS were quantified from C57BL6/J wild type and APP−/− mice. (A) To assess stool production and water absorption, wet and dry stool weights and the differences (water content) were collected over 1hr. Data from 13 animals in each group are displayed as mean (+/−SD). (B) To assess animal weight difference between strains, mice were weighed prior to collection. (C) To quantify uptake/transport of intestinal LPS into blood, whole blood was collected, plasma was separated and the LAL Endotoxin assay was performed according to manufacturer protocol. Data from 5 animals in each group are displayed as mean (+/−SD).*p<0.05, ***p<0.001.
