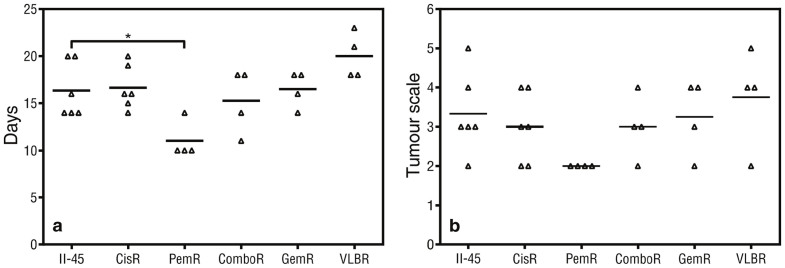
Figure 3. Endpoint data for rats with pleural mesothelioma.
Rats were injected with 5 × 105 parental or chemo-resistant II-45 cells directly into the pleural cavity. (a) Mean time (days) until ethical endpoint (weight loss of >10% or difficulty in breathing); (b) Pleural tumor scale at endpoint in rats with mesothelioma. P-value was calculated using a one-way Anova test with a value of less than 0.05 indicating significance. *p < 0.05 relative to rats with II-45 mesothelioma cells. Parental mesothelioma cells, II-45; cisplatin resistant II-45 cells, CisR; pemetrexed resistant II-45 cells, PemR; combination (cisplatin + pemetrexed) resistant II-45 cells, ComboR; gemcitabine resistant II-45 cells, GemR; vinorelbine resistant II-45 cells, VLBR.